|
|
|

|
|
|
|
What ARE Turmeric and Curcumin? |
|
Turmeric is a spice obtained
from the dried root rhizomes of Curcuma longa, a
medicinal plant from the ginger family of
herbs, the Zingiberaceae. The rhizomes are dried and crushed in order to produce a yellow-orange powder. The biologically active compound
within this powder is curcumin, one of the most studied
phytochemicals in science. Turmeric powder contains
2% to 8% curcumin, which
we isolate to 95% for medical research and nutritional supplementation. |
|
Where
to purchase Curcumin? |
|
Since 2000,
TURMERIC-CURCUMIN.COM has
offered Curcumin 95% extract
supplements to research institutions, physicians, and university medical centers. Quality control tests, laboratory analysis
certification, and rigorous
cGMP manufacturing
standards all ensure freshness, potency, and purity of contents. Orders are shipped
FedEx
or USPS Priority Air for fast and secure delivery. Contact
service@turmeric-curcumin.com
for bulk ordering, private
labeling, contract manufacturing or any other questions. Multiple bottle orders
will receive increasing quantity discounts listed below. Case purchases (12
bottles) will also
receive free US shipping. |
|
 |
.
| |
|
|
|
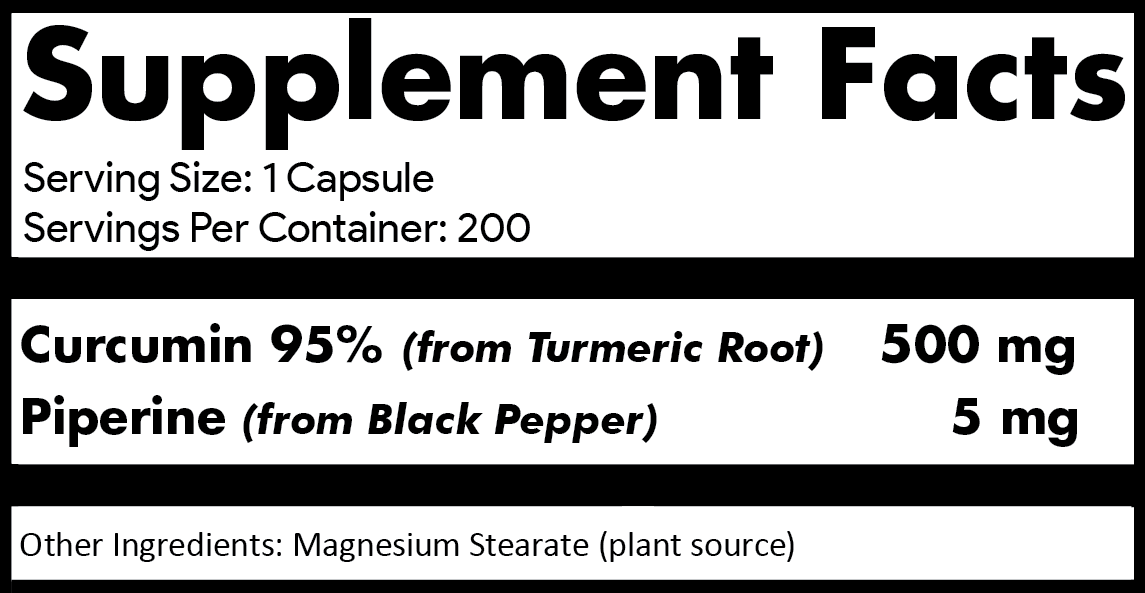
why 200
capsules per bottle and
500
mG
OF Curcumin
95%
per capsule?
|
|
Our label clearly
indicates the
contents (curcumin
extracted from turmeric root),
purity (95% standardized),
amount
per capsule (500mg) and number of
capsules per bottle (200). Each 12-bottle case contains
over 2.6 lbs (1.2 kg) of curcumin 95% turmeric root extract.
Since 2000, the geographical source of our curcumin
extract is turmeric grown in India. Within India, we are
extensively networked with selected pesticide-free farms in the
Eastern Ghats highlands, known for
turmeric with the highest natural
curcumin
content in the world, at 8.8%
and
the West Jaintia Hills of Meghalaya, India, where Lakadong
turmeric is one of the world's finest with a curcumin content of
about 7.5%. From this high quality
turmeric (Curcuma
longa) root, we extract curcumin to a minimum 95%
concentration. Within this extraction is the full spectrum of curcuminoids -
curcumin, demethoxycurcumin, and bisdemethoxycurcumin - in their
natural composition ratio of 76:19:5 for maximum potency. This is the
same extract
used
in clinical trials and medical studies,
free of added chemicals, treatments, unknown "formulas",
"complexes" or "proprietary blends" which do not even disclose
how much of each ingredient the product contains. Our supplement
contains just two active ingredients; 500mg Curcumin 95% and 5mg
of Piperine.
You will receive a
100% natural, additive-free product.
There are no
synthetics, no starch, no sugars or sweeteners, no artificial colors or flavors, no sodium, no soy,
no yeast, no wheat, no corn, no rice or other grains, no gluten, no dairy, no preservatives, no
gums, no dyes, and no GMO. |
|
HAS THE SAFETY OF CURCUMIN
95%
BEEN ESTABLISHED?
|
|
The safety, tolerability, and
nontoxicity of curcumin at high doses has been well
established by human clinical trials.
The
US FDA classifies Turmeric as
GRAS (Generally
Recognized As Safe). Preclinical
and clinical studies indicate that curcumin is well tolerated
and the overwhelming safety profile of curcumin is evident.
Before using any herbs or dietary supplements in amounts greater
than usually found in foods, consult a health care provider.
"Clinical studies have shown that curcuminoids, including
curcumin, bisdemethoxycurcumin, and demethoxycurcumin, have
safety characteristics at daily doses of 4000–8000 mg at 95%
concentration. The US Food and Drug Administration (FDA) has
given curcumin the " generally recognized as safe" (GRAS)
designation. Various studies have shown that curcumin is safe,
well-tolerated, and useful in the prevention and treatment of a
variety of chronic diseases, such as cancer, heart disease,
diabetes, neurological disorders, skin conditions, liver
complications, and infectious diseases." -
Biomedicine & Pharmacotherapy | 2024
"Long-term studies have shown that curcumin is safe and
protective when used in the diet. In one study, high doses of
curcumin (8 g/day) do not cause side effects. In another study,
curcumin was administered at doses up to 12 g/day for three
months with no apparent toxicity." -
Review of the Protective Mechanism of Curcumin on Cardiovascular
Disease | 2024
"As
an antioxidant, anti-infection, anti-inflammatory, and
anti-tumor compound, curcumin has been approved by the United
States Food and Drug Administration as a safe compound." -
Molecular Neurobiology | 2024
"Curcumin has been demonstrated to be safe even when it is
administered at high doses. A phase 1 human trial in which as
much as 8000 mg of curcumin per day was administered for 3
months to patients with high-risk or premalignant lesions
reported no toxic effects. No serious side effects were reported
in RA patients receiving 500 mg of curcumin per day over a
period of 8 weeks. Curcumin treatment has no obvious toxic
effect on liver or kidney functions; therefore, curcumin is
generally recognized as a safe compound by the U.S. Food and
Drug Administration." -
Frontiers in Pharmacology | 2024
"The European Food Safety Authority (EFSA) established
an acceptable daily intake of curcumin at 3 mg/kg body weight.
For optimal pharmacological effects, an oral dose of more than
8.0 g/day is often required. Numerous clinical studies
demonstrated that a daily intake of 12 g of curcumin is well
tolerated and safe." -
Pharmaceutics |
2024
"Curcumin has been used as a dietary supplement for centuries
and is considered pharmacologically safe." -
International Journal For Multidisciplinary Research | 2024
"A series of authoritative international institutions, such as
the Food and Drug Administration (FDA) in the USA and the Joint
FAO/WHO Expert Committee on Food Additives, have confirmed the
safety of curcumin in daily use and clinical treatment." -
Toxics | 2023
"According to the Joint Nations and World Health Organization
Expert Committee on Food Additives (JECFA), curcumin is regarded
as a safe chemical and is hence appropriate for everyday dietary
usage and can be taken by patients of any age for a longer
period of time without experiencing any negative side effects."
-
Biomedicine |
2023
"Curcumin is one of the most promising anticancer agents as it
combines high biological safety for normal cells/tissues with
potent cytotoxic activity against various human cancers." -
International Journal of Molecular Sciences | 2023
"According to the US Food and Drug Administration (FDA) report,
curcumin has been considered as “Generally Recognized as Safe”
(GRAS) even at doses between 4000 and 8000 mg/day" -
Evidence-Based Complementary and Alternative Medicine | 2023
"The safety of curcumin and turmeric products has been confirmed
by the Food and Drug Administration (FDA), the Food and
Agriculture Organisation (FAO) and the World Health Organisation
(WHO). Curcumin has shown a very promising safety profile.
According to reports by JECFA (Joint Expert Committee on Food
Additives of the United Nations and the World Health
Organisation) and EFSA (European Food Safety Authority), the
acceptable daily intake (ADI) of curcumin is 0–3 mg/kg body
weight. Several studies in healthy volunteers have confirmed the
safety and efficacy of curcumin." -
An Overview
of the Enhanced Effects of Curcumin and Chemotherapeutic Agents
in Combined Cancer Treatments | 2023
"Curcumin has also been recognized as safe by the US Food and
Drug Administration (FDA). In most studies, very few adverse
effects or no severe adverse effects occurred with turmeric
extract and curcumin supplements, showing that it’s well
tolerated globally. None of the patients required rescue
medication for adverse effects management. Overall, the benefits
of Curcuma longa extract and curcumin supplementation
were significantly greater than their risks. Therefore, it can
be recommended for musculoskeletal conditions." -
Safety and Efficacy of Turmeric (Curcuma longa) Extract and
Curcumin Supplements in Musculoskeletal Health | 2023
"Curcumin has been shown to be safe in numerous human studies,
with only minor toxicity associated with this polyphenol.
Therefore, curcumin is increasingly being viewed as a
biomolecule capable of being administered for an extended period
without causing adverse effects. The findings of the current
study indicate that there were no significant adverse events
associated with the short term use of PPI and curcumin." -
BMJ British Medical Journal | 2023
"Curcumin is labeled as safe by the Food and Drug Administration
(FDA, USA), and has achieved therapeutic pursuit in treating
metabolic diseases, immune-related diseases, and cancer, owing
to its vast biological target and with practically no
aftereffects. Curcumin is an active natural compound that
exhibits therapeutic effects on different diseases, including
antiviral, antibacterial, anti-amyloid, thrombo-suppressive,
antiarthritic, antioxidation, anti-inflammatory, and anticancer,
with minimal aftereffects. Curcumin is safe in humans and has
chemopreventive and chemotherapeutic effects. In vitro
experimental evidence indicates that curcumin has excellent
anticancer ability and can target cancer via diverse mechanisms
by modulating several cellular signal pathways." -
Curcumin a
Natural Phenol and Its Therapeutic Role in Cancer | 2023
"Genotoxicity and mutagenicity assessments suggest that
curcumin extract does not induce DNA damage or mutations.
Furthermore, carcinogenicity studies demonstrate no evidence of
increased cancer risk associated with the curcumin extract." -
Department of Nutrition, Central Michigan University | 2023
"The Joint Food and Agriculture
Organization (FAO), World Health Organization (WHO) Expert
Committee on Food Additives (JECFA) and the European Food Safety
Authority (EFSA) allocated an acceptable daily intake (ADI) for
curcumin of 3 mg/kg body weight." -
Nutrients |
2022
"Curcumin is considered a safe
compound and authorized as a GRAS compound (generally recognized
as safe) by US FDA (United States Food and Drug Administration).
It is well tolerated at a higher dose of 12g in humans. Cells
treated with the curcumin-piperine combination at their EC90
concentration showed no toxicity to neuronal cells. Reduction in
locomotive behaviors was not observed with the curcumin-piperine
combination, indicating no potential CNS side effects of
curcumin-piperine combination at its highest therapeutic doses.
We found no effects of the combination on the spontaneous
locomotor activity at their high doses. The results indicate no
potential central nervous system (CNS) side effects of the
curcumin and piperine combination." -
CM Journal | 2022
"Curcumin is “generally recognized as safe” (GRAS) as a dietary
supplement by the U.S Food and Drug Administration (FDA) and the
European Food Safety Authority (EFSA) and has been catalogued
with the E100 code of the European Union." -
Curcumin: A
Novel Way to Improve Quality of Life for Colorectal Cancer
Patients? International Journal of Molecular Sciences | 2022
"According to the US Food and Drug Administration, curcumin is
classed as safe for both human consumption and pharmacological
purposes without any known side effects." -
Life |
2022
"Studies have shown that systemic exposure to
curcumin-containing products at doses of up to 8,000 mg/day was
safe and tolerable and did not cause serious adverse events." -
Curcumin (Curcuma, Turmeric) and Cancer, PDQ Integrative,
Alternative, and Complementary Therapies Editorial Board | 2022
"According to relevant clinical trials on
safety and toxicity, the acceptable dose of curcumin for maximum
efficacy is 4 – 8 grams per day. It has been reported that
humans can tolerate treatment with curcumin at a dose up to 12 grams
per day." -
Frontiers in Pharmacology | 2022
"The US Food and Drug Administration has marked curcumin as a
"By and large Recognized As Safe" item. The United Nations and
World Health Organization Expert Committee on Food Additives and
European Food Safety Authority suggested the everyday admission
of 0-3 mg/kg body weight of curcumin. Furthermore, curcumin
supplementation in a few clinical preliminaries exhibited
wellbeing profiles at dosages in the range of 4000 and 8000 mg/
day." -
Journal of Antimicrobial Agents, Prevention of Female
Reproductive Disorders with the Help of Curcumin | 2022
Archived studies on
the safety of curcumin |
|
does piperine improve absorption, enhance
bioavailability, and work synergistically with Curcumin?
|
"The association with piperine, an alkaloid derived from black
pepper (Pipernigrum L), is capable of increasing the
bioavailability of some drugs by inhibiting intestinal and
hepatic glucuronidation. In humans, administration of 20mg of
piperine with 2g of curcumin increased its bioavailability by
2000% compared to administration of 2g of curcumin alone. When
curcumin (20 and 40mg/kg) was co-administered with piperine
(bioavailability enhancer) at a dose of 2.5mg/kg, its
pharmacological effects were intensified. Piperine is capable of
increasing absorption, plasma concentration, and bioavailability
of curcumin in both rats and humans without significant side
effects. The administration of 20mg/kg of piperine with 2g/kg of
curcumin in rats increases its bioavailability by 154% compared
to administration of 2g/kg of curcumin alone. Piperine is a
non-specific drug metabolism inhibitor, with low discrimination
between different forms of cytochrome P-450. In rats, orally
administered piperine strongly inhibits the hepatic activity of
aryl hydrocarbon hydroxylase (AHH) and UDP-glucuronyl
transferase, with a potent inhibitory effect on pharmacological
metabolism." -
Curcumin in Alzheimer’s Disease and Depression: Therapeutic
Potential and Mechanisms of Action | 2024
“Piperine, the active compound in black pepper, can enhance
curcumin absorption (from turmeric) by up to 2,000%. This
synergy not only amplifies the benefits of curcumin for the
brain but also helps in improving digestion and nutrient
absorption, indirectly supporting cognitive health.” -
NY Post | 2024
"Combining piperine with curcumin has been shown to increase
curcumin bioavailability in humans." -
Food Chemistry | 2024
"Piperine, a bioactive
compound from black pepper (Piper nigrum), emerges as a
potentiation agent capable of inhibiting hepatic
glucuronidation, thereby increasing curcumin bioavailability by
up to 2000%. The combination of curcumin and piperine has shown
successful outcomes in diseases where oxidative stress is a
significant etiological factor, such as metabolic syndrome.
Curcumin plus piperine group had significantly higher serum
superoxide dismutasecompared to the placebo group. This study
suggests that 12 weeks of curcumin plus piperine supplementation
effectively enhances the enzymatic antioxidant defense in IBD
patients. The results provide new insights into the use of a
natural therapeutic strategy and underscore the effectiveness of
its combination with piperine, in enhancing the antioxidant
effects of curcumin." -
Effect of Curcumin plus Piperine on Redox Imbalance and
Inflammation in Inflammatory Bowel Disease Patients | 2024
"Curcumin and piperine supplementation before and after
exercise positively affects the muscle damage of athletes after
exercise." -
Examination of the effect of curcumin supplementation on liver
enzymes and some physiological parameters in volleyball players
| 2024
"Curcumin plus piperine administration
showed a significantly increased superoxide dismutase activity
and glutathione levels while significantly decreased
malondialdehyde concentrations. In addition, our study revealed
that curcumin plus piperine significantly decreased tumor
necrosis factor-alpha (TNF-alpha) and interleukin-6
concentrations." -
The
Effects of Curcumin Plus Piperine Co-administration on
Inflammation and Oxidative Stress | 2024
"The bioavailability of curcumin can be enhanced by piperine (an
alkaloid derived from black pepper). Using piperine combined
with curcumin significantly increased serum levels of curcumin
in humans and animals by 2,000 times because of the extensive
absorption and bioavailability." -
The Use of Curcumin in the Treatment of Colorectal, Breast,
Lung, and Prostate Cancers: An In Vivo study Update | 2024
"The combination of curcumin and piperine has shown
successful outcomes in diseases where oxidative stress is a
significant etiological factor, such as metabolic syndrome. The
results underscore the effectiveness of combination with
piperine in enhancing the antioxidant effects of curcumin.
Piperine, a bioactive compound from black pepper (Piper nigrum),
emerges as a potentiation agent capable of inhibiting hepatic
glucuronidation, thereby increasing curcumin bioavailability by
up to 2000%. The curcumin plus piperine group had significantly
higher serum SOD compared to the placebo group. This study
suggests that 12 weeks of curcumin plus piperine supplementation
effectively enhances the enzymatic antioxidant defense,
particularly SOD, in inflammatory bowel disease patients." -
Effect of Curcumin plus Piperine on Redox Imbalance and
Inflammation in Inflammatory Bowel Disease Patients | 2024
"When analyzed in humans, 2 grams of isolated curcumin
showed undetectable or very low serum levels. After the
concomitant administration of 20 mg of piperine, an increase in
concentrations was observed within the time frame of 0.25 to 1
hour after administration. In those rats in which piperine
pre-administration was performed before receiving curcumin,
there was a significant increase in the oral bioavailability of
curcumin, especially at 6 hour after piperine administration.
Piperine, when administered concomitantly with 2 grams of
curcumin in healthy human volunteers, increased the curcumin
bioavailability by 2000%. Curcumin and piperine supplementation
for obese mice under caloric restriction may increase the loss
of body fat and suppresses HFD-induced inflammation." -
Antioxidants |
2024
"Both curcumin and piperine suppress proliferation of
leukemia cells and their IC50 value has reported to be 30 μM and
25 μM, respectively. These anti-cancer agents have capacity of
inducing apoptosis in leukemia cells via mitochondrial pathway.
Besides, curcumin and piperine induce autophagy and mediate S
arrest.The current section clearly demonstrated that curcumin
and its combination with other therapies can suppress leukemia
progression." -
Journal of Herbal Medicine, The Effects of Curcumin on
Neurodegenerative Diseases: a Systematic Review | 2024
"To improve the bioavailability of curcumin, one of the
potential strategies is adding piperine when administering
curcumin orally. Piperine also has antioxidant,
immunomodulatory, and anti-inflammatory activities. Piperine can
increase the in vivo bioavailability of curcumin by
inhibiting its metabolism and reducing the required dose of
curcumin in the clinical setting. Piperine binds to several
areas of the enzyme to form a hydrogen bond complex with
curcumin that can increase its bioavailability up to twenty
times." -
Vitamin D
and Curcumin Piperine Attenuates Disease Activity and Cytokine
Levels in Systemic Lupus Erythematosus Patients | 2024
"Studies have shown that combining curcumin with piperine, a
compound found in black pepper, can increase its bioavailability
by inhibiting metabolism." -
PLoS One | January 2024
"Black pepper (Piper nigrum) family piperaceae,
with the main active ingredient piperine, has a hypoglycemic
effect (Panda & Kar, 2003). Turmeric (Curcuma longa),
family Zingiberaceae, in which a vital active ingredient is
curcumin, has been revealed to have hypoglycemic, antioxidant,
and lipid-lowering effects in many investigational studies (Khaliq
et al., 2015). Earlier studies revealed that both herbs work in
synergy in lowering postprandial blood glucose levels It was
hypothesized that black pepper and turmeric together have a
greater effect on lowering postprandial glycemia." -
A
Review on the Extraction Process and Therapeutic Activity of
Curcumin on Diabetes Mellitus and Cancer | 2024
"Piperine enhances bioavailability when combined with
curcumin in a complex. This was connected previously with 2000%
increase in curcumin bioavailability. Several organic compounds
have also been utilized to boost curcumin bioavailability, the
majority of which reduce the metabolism of curcumin and enhance
its absorption. Most of these compounds were developed to slow
down the metabolism of curcumin and improve its bioavailability.
Piperine, the primary active component of black pepper, is the
most widely used." -
Biomedicine & Pharmacotherapy | 2024
"Piperine
has been found to increase the bioavailability of curcumin
significantly. Curcumin (oral, 1 g/d plus piperine 10 mg/day for
12 weeks) reduced cardiovascular risk and enhanced antioxidant
capacity in type 2 diabetes mellitus through decreasing serum
levels of total cholesterol and non-HDL-cholesterol." -
Pharmacological Research | 2023
"Piperine increased the bioavailability of curcumin by
154%. Curcumin combined with piperine exhibited higher
intestinal absorption (78%). When co-administered with piperine,
the half-life of curcumin was increased from 12.8h to 28.9h.
Co-administration of piperine (20 mg/kg) and curcumin (2 mg/kg)
increased the plasma concentration of curcumin in a short time,
i.e. within 1-2h, peak time was increased, elimination half-life
decreased, and clearance decreased. In humans, even with a
2g load of curcumin, the serum level was undetectable. A higher
extent of absorption and higher bioavailability of curcumin
(2000%) was observed in humans using 20mg piperine." -
Review of Curcumin and Its Different Formulations:
Pharmacokinetics, Pharmacodynamics and
Pharmacokinetic-Pharmacodynamic Interactions | 2023
"Piperine is known to enhance curcumin absorption and tissue
uptake, while also reducing curcumin hepatic metabolism. Once
absorbed, curcumin is metabolized within the epithelial cells
and/or effluxed back into the intestinal lumen. This efflux is
thought to occur due to the presence of efflux transporters
within the intestinal cell membranes. Piperine from black
pepper, as well as certain catechins from green tea, are able to
inhibit these transporters, thus increasing the amount of
curcumin that remains in the body. A preclinical study
administrated an orally delivered powder of curcumin (2
g/kg/day) and piperine (20 mg/kg/day) and concluded that
piperine significantly increases the serum concentration of
curcumin, resulting in a 154% improvement in its relative
bioavailability. Similarly, a clinical study conducted with the
combined oral administration of curcumin and piperine dissolved
in water (2 g of curcumin powder, 20 mg of piperine and 150 mL
of water). The study found that piperine significantly increased
the serum concentration of curcumin, resulting in a relative
bioavailability increase of 2000%" -
Effect of Curcumin Consumption on Inflammation and Oxidative
Stress in Patients on Hemodialysis: A Literature Review | 2023
"The bioavailability of curcumin was significantly increased
by 2000% when piperine, which is extracted from black pepper,
was also taken as a supplement." -
Effect of
Curcumin and Coenzyme Q10 Alone and in Combination on Learning
and Memory in an Animal Model of Alzheimer’s Disease | 2023
"Piperine is an adjuvant that can greatly improve
bioavailability of curcumin. Piperine slows the metabolism of
curcumin by inhibiting hepatic and intestinal glucuronidation.
Previous studies have shown that administering curcumin with
piperine can increase serum concentrations of curcumin by up to
2000%, indicating that glucuronidation inhibition may be the
major mechanism of increasing curcumin bioavailability. As such,
the formulation of the supplement administered in the current
study, 1,400 mg of curcumin and 10 mg of piperine, may have
helped to increase serum concentrations of curcumin such that it
could exert its biological action." -
Frontiers in Nutrition, Sport and Exercise Nutrition | 2023
"The study has found that curcumin (oral, 1 gram per day
plus piperine 10 mg/day for 12 weeks) reduced cardiovascular
risk and enhanced antioxidant capacity in T2DM through
decreasing serum levels of TC, non-HDL-c and MDA, while also
increasing serum adiponectin, SOD or HDL-C levels." -
Pharmacological Research | 2023
"The combination of curcumin with piperine has better
gastrointestinal absorption and reduces curcumin's systemic
excretion. Piperine increases the bioavailability of curcumin by
binding to the enzyme glucuronidase in the intestine, preventing
glucuronidation and reducing the excretion of curcumin from the
stool" -
The effect of curcumin-piperine on cardiometabolic, inflammatory
and oxidative stress factors and macular vascular density | 2023
"Curcumin + piperine decreased waist circumference, systolic
blood pressure, total cholesterol, low-density
lipoprotein-cholesterol, fasting blood glucose, alanine
transaminase and aspartate transaminase compared with placebo.
Curcumin + piperine may be considered as an adjunct therapy to
improve anthropometric measures, blood pressure, lipid profile,
blood glucose, and liver function in Non-alcoholic Fatty Liver
Disease patients." -
Efficacy of
curcumin plus piperine co-supplementation in moderate-to-high
hepatic steatosis | 2023
"Curcumin-piperine
supplementation for 12 weeks resulted in significant reductions
in serum levels of total cholesterol, triglycerides, weight,
waist circumference, and systolic and diastolic blood pressure
in patients. To improve pharmacokinetic features,
co-administration of curcumin with piperine has been introduced
as an alternative. Piperine, which is a naturally occurring
alkaloid from pepper, has been shown to increase the
bioavailability of curcumin and reduce its glucuronidation." -
The effects of curcumin-piperine supplementation on
inflammatory, oxidative stress and metabolic indices in patients
with ischemic stroke in the rehabilitation phase: a randomized
controlled trial | 2023
"Recent studies
demonstrated that piperine potentiates curcumin’s inhibitory
effect on tumor progression via enhancing its delivery and
therapeutic activity. Among the numerous candidates tested so
far, curcumin, piperine and certain types of cannabinoids
performed promisingly well in colon carcinoma models as
monotherapy agents. Piperine, a dietary polyphenol isolated from
black and long peppers, distinguished with its intrinsic
features, improves not only curcumin’s existing anti-cancer
activity, but also its extremely poor bioavailability. As a
single agent, piperine alone also displays anti-mutagenic and
anti-tumor activities." -
Frontiers in Pharmacology | 2023
"Several
studies done by using curcumin in conjunction with various
anticancer compounds, as in piperine...have shown significant
suppression, reduction/inhibition of IL-6, IL-1β, IL-19, TNF-α
and COX-2 (Al-Dossari et al., Citation2020; X. Q. Hu et al.,
Citation2016; Neyrinck et al., Citation2013; Tremmel et al.,
Citation2019; Yan et al., Citation2019)." -
Curcumin: recent updates on gastrointestinal cancers | 2023
"Black pepper piperine is one of the most effective boosters
of curcumin bioavailability. The simultaneous administration of
curcumin and piperine to humans or animals boosted the serum
levels by more than a thousandfold. Piperine contained in black
pepper can improve the uptake of curcumin by 2,000% (20 times).
The vast metabolism of turmeric in the hepatic tissues and
intestinal walls increased its bioavailability, which improved
through piperine. Taking these two substances with an oil rich
in unsaturated fatty acids further strengthens this benefit.
Zeng et al. examined the effect of piperine pre-administration
on oral curcumin bioavailability. In this investigation, rats
were given 20 mg/kg piperine first, followed by 200 mg/kg
curcumin at intervals of 0.5–8 h after piperine treatment. The
pre-treatment with piperine before curcumin administration
significantly increased curcumin oral bioavailability in all
tested rats. Recent research suggests that oral administration
of curcumin and piperine for symptomatic COVID-19 therapy might
dramatically reduce mortality and morbidity (53). The
conjugation between piperine and curcumin may be a safe and
natural option for preventing post-COVID symptoms." -
Impacts of turmeric and its principal bioactive curcumin on
human health: Pharmaceutical, medicinal, and food applications:
A comprehensive review | 2023
"The combination
of Curcumin with piperine was associated with a 2000%
improvement in Curcumin bioavailability." -
Health Science Reports | 2023
"Another
promising approach is the simultaneous administration of
curcumin with piperine, an alkaloid from black pepper and long
pepper. Piperine significantly increases the bioavailability of
curcumin—up to 2000%—by preventing its metabolism." -
An Overview
of the Enhanced Effects of Curcumin and Chemotherapeutic Agents
in Combined Cancer Treatments | 2023
"Curcumin has
received worldwide attention for its multiple health benefits,
which are best achieved when curcumin is combined with agents
such as piperine to increase its bioavailability significantly.
Pawar et al reported that the administration of oral curcumin
with piperine as symptomatic adjuvant therapy in COVID 19
treatment could substantially reduce morbidity and mortality and
ease the logistical and supply related burdens on the healthcare
system. Askari et al reported that 46 outpatients with COVID 19
disease were randomly allocated to receive two capsules of
curcumin - piperine for 14 days. There was a significant
improvement in dry cough, sputum cough, ague, sore throat,
weakness, muscular pain, headache and dyspnoea in curcumin -
piperine groups. Kumar et al reported that using curcumin, Piper
Nigrum Piperine and catechin could cure and prevent COVID 19
outbreaks and infection. Curcumin and piperine interact and form
a π–π intermolecular complex, which enhances curcumin's
bioavailability." -
Therapeutic potential of curcumin in ARDS and COVID 19 | 2023
"Curcumin by itself has very low bioavailability due to
ineffective absorption, and fast metabolism and excretion. This
issue can be solved by combining curcumin with piperine (a key
active ingredient in black pepper) to create a curcumin complex
that is readily absorbed and metabolized in the body. Curcumin
is more active when combined with piperine to provide major
health benefits. These benefits are maximized when curcumin is
coupled with agents such as piperine, that significantly
increase its bioavailability." -
The Therapeutic Role of Curcumin in Inflammation | 2023
"Curcumin has received worldwide attention for its multiple
health benefits, which appear to act primarily through its
anti-oxidant and anti-inflammatory mechanisms. These benefits
are best achieved when curcumin is combined with agents such as
piperine, which increase its bioavailability significantly." -
Department of Nutrition, Central Michigan University | 2023
Archived studies on curcumin and piperine |
|
What is the suggested usage and dosage for curcumin
95%?
|
|
"For optimal pharmacological effects, an oral dose of more than
8.0 grams per day is often required. Numerous clinical studies
demonstrated that a daily intake of 12 grams of curcumin is well
tolerated and safe." -
Pharmaceutics |
2024
"Patients taking curcumin once or
twice a day reported significant symptom improvement compared to
patients taking it sporadically." -
Rheumatology International | 2024
"Researchers concluded that if you want to receive the maximum benefits
from curcumin, 30 mg per kilogram body weght is the most effective dose." -
Molecular Neurobiology | 2024
"Curcumin's protective effect is
proportional to the dose, and the efficacy may be further
increased at a concentration of more than 200 mg per kg. Results
indicated that the efficacy of curcumin improved with higher
administered doses within the concentration range of 200 mg per
kg. The results from the fitted curves suggest that efficacy may
further improve at concentrations exceeding 200 mg/kg." -
Cardioprotective Effects of Curcumin Against Diabetic
Cardiomyopathie | 2024
"A phase I trial examining the impact
of curcumin on advanced colorectal cancer did not detect
curcumin concentration in the plasma at lower doses. However,
metabolites of curcumin were identified in plasma at higher
doses." -
Food Chemistry | 2024
"In a study on curcumin treatment
of drug-resistant tumor cells, a low dose of curcumin showed no
effect on antioxidant proteins, whereas a high dose resulted in
the inhibition of antioxidant proteins. Furthermore, high-dose
curcumin treatment has been reported to exacerbate the effects
on damaged mitochondria. This results in mitochondrial and DNA
damage and subsequent activation of the cell death pathway,
providing possible approaches for cancer therapy." -
Oncology Letters | 2024
"A study used two
dosages (2000 mg and 4000 mg) of curcumin powder daily for 30
days in 40 participants, aiming to decrease procarcinogenic
factors. Only the higher dose group showed a 40% reduction in
these foci, correlating with a significant increase in plasma
curcumin levels." -
Curcumin in
Cancer and Inflammation | 2024
"Curcumin
supplementation in doses of 3000 mg/day over 8–12 weeks showed
reductive effect on total cholesterol levels, however curcumin
therapy with doses less of 1000 mg per day has had no
significant effect." -
Complementary Therapies in Medicine Volume | 2024
"The US Food and Drug Administration
has permitted curcumin's safety. Because of multiple medical
research on the safety and harmfulness of curcumin, a tolerable
dosage of 4 – 8 grams per day is regarded to achieve the best
therapeutic results" -
Food and Agricultural Immunology | 2023
"The dose-dependent action of curcumin observed in our results
highlights the importance of considering its concentration in
cancer treatment. We found that increasing concentrations of
curcumin led to a proportional decrease in cell viability,
migration, and invasion in gastric cancer cells. This suggests
that the efficacy of curcumin in inhibiting cancer cell
progression is influenced by its dose, with higher
concentrations resulting in more pronounced effects. The
dose-dependent response may be attributed to curcumin's complex
interactions with multiple cellular targets and signaling
pathways. At lower concentrations, curcumin may predominantly
target specific pathways, while higher concentrations may engage
multiple pathways, leading to a more potent inhibitory effect."
-
Dose-Response | 2023 |
|
Why NATURAL curcumin 95%
extract from turmeric root without nanoparticles or
other highly processed synthetics, liposomals or emulsifiers? |
"The use
of curcumin in liposomal form is becoming more and more common,
the concentration of this compound, in cells can reach much
higher level, definitely exceeding the amounts that can enter
the body when applied in natural form. The conducted experiments
clearly showed that liposomal curcumin, when directly applied to
cells, is toxic to them. Comparison of the toxicity of curcumin
for HL-60 cells and nerve cells confirmed (as shown in model
studies) that the disruption of membrane structure due to the
presence of liposomal curcumin correlates with damage to native
membranes, ultimately leading to cell death." -
Scientific Reports | 2024
"In the
last few years questions have been raised regarding the
potential toxicity of carbon nanotubes (CNTs) to humans and
environment. It is believed that the physico-chemical
characteristics of these materials are key determinants of CNT
interaction with living organisms, and hence determine their
toxicity." -
Determinants of carbon nanotube toxicity | 2023
"This
study evaluated the toxic events of curcumin nanoparticles with
alterable surface polarity in alveolar macrophages. In
conclusion, the cytotoxicity of curcumin nanoparticles on
alveolar macrophages is surface-charge dependent, which in turn
is associated to the uptake pathway and localization of curcumin
nanoparticles in cells." -
Toxicity of curcumin nanoparticles towards alveolar macrophage,
Food and Chemical Toxicology | 2022
"The
harmfulness of nanoparticles is impacted by their condition of
conglomeration and mechanical properties, which are reliant upon
their creation and decontaminating strategies. Worries about the
poisonousness of nanoparticle-based conveyance strategies
incorporate neuroinflammation, excitotoxicity, and unfavorably
susceptible responses." -
Alternative & Integrative Medicine | 2022
"Curcumin
nanomicelle suppressed spermatogenesis, increased
immunoreactivity of 8-oxodG, stimulated the Hsp70–2a and Hsp90
expressions, and resulted in severe DNA and mRNA damages.
Moreover, the curcumin nano-micelle received animals exhibited
remarkable reductions in the spermatozoa count, motility and DNA
integrity. In conclusion, chronic and high dose consumption of
curcumin nanomicelle results in remarkable oxidative stress." -
Curcumin nano-micelle induced testicular toxicity in healthy
rats; evidence for oxidative stress, Biomedicine &
Pharmacotherapy | 2021
"A number of
nanoparticles have negative impacts on male germ and somatic
cells which could ultimately affect fertility or the ability to
produce healthy offspring." -
Toxicity mechanisms of nanoparticles in the male reproductive
system | 2021
"Nanoparticles may act as
reproductive toxicants depending on several factors, and induce
damage to the male reproductive system by affecting the
seminiferous tubules and spermatogenesis. This is mainly due to
the fact that nanoparticles can easily enter the blood
circulatory system and reach the testes by crossing the blood
testes barrier. The bioaccumulation of nanoparticles in the
testes causes seminiferous tubule histopathology and severely
affects the sperm number, motility and morphology. Moreover,
nanoparticles also induce disturbances to the Leydig cells,
causing decline in the testosterone level with consequent
testicular injury and reduced sperm production." -
Perspectives of Nanoparticles in Male Infertility: Evidence for
Induced Abnormalities in Sperm Production | 2021
"Curcumin nanoparticles suppressed the proliferation of
testicular cell lines in vitro. In the
present study, we disclosed the acute damage on mouse
spermatogenesis and sperm parameters by nano-curcumin. Our
results suggested that the reproductive toxicity of
nanoformulated curcumin needs to be prudently evaluated before
its application." -
Acute Damage to the Sperm Quality and Spermatogenesis in Male
Mice Exposed to Curcumin-Loaded Nanoparticles, International Journal of
Nanomedicine | 2020
"Nanoparticles are able to pass
certain biological barriers and exert toxic effects on crucial
organs, such as the brain, liver, and kidney. Only recently,
attention has been directed toward the reproductive toxicity of
nanomaterials. Nanoparticles can pass through the blood–testis
barrier, placental barrier, and epithelial barrier, which
protect reproductive tissues, and then accumulate in
reproductive organs. nanoparticles accumulation damages organs
(testis, epididymis, ovary, and uterus) by destroying Sertoli
cells, Leydig cells, and germ cells, causing reproductive organ
dysfunction that adversely affects sperm quality, quantity,
morphology, and motility or reduces the number of mature oocytes
and disrupts primary and secondary follicular development. In
addition, nanoparticles can disrupt the levels of secreted
hormones, causing changes in sexual behavior. However, the
current review primarily examines toxicological phenomena. The
molecular mechanisms involved in nanoparticles toxicity to the
reproductive system are not fully understood, but possible
mechanisms include oxidative stress, apoptosis, inflammation,
and genotoxicity. Previous studies have shown that nanoparticles
can increase inflammation, oxidative stress, and apoptosis and
induce ROS, causing damage at the molecular and genetic levels
which results in cytotoxicity." -
Potential adverse effects of nanoparticles on the reproductive
system
"Recent studies have shown that
nanoparticles disturb the developing oocyte by invading the
protective barrier of theca cells, granulosa cell layers and
zona pellucida. Nanoparticles disrupt sex hormone levels through
the hypothalamic–pituitary-gonadal axis or by direct stimulation
of secretory cells, such as granule cells, follicle cells,
thecal cells and the corpus luteum. Some nanoparticles can cross
the placenta into the fetus by passive diffusion or endocytosis,
which can trigger fetal inflammation, apoptosis, genotoxicity,
cytotoxicity, low weight, reproductive deficiency, nervous
damage, and immunodeficiency, among others." -
Nanoparticles and female reproductive system: how do
nanoparticles affect oogenesis and embryonic development
"Females are particularly more
vulnerable to nanoparticle toxicity, and toxicity in this
population may affect reproductivity and fetal development.
Moreover, various types of nanoparticles have negative impacts
on male germ cells, fetal development, and the female
reproductive system." -
Toxicity of Nanoparticles on the Reproductive System in Animal
Models: A Review |
|
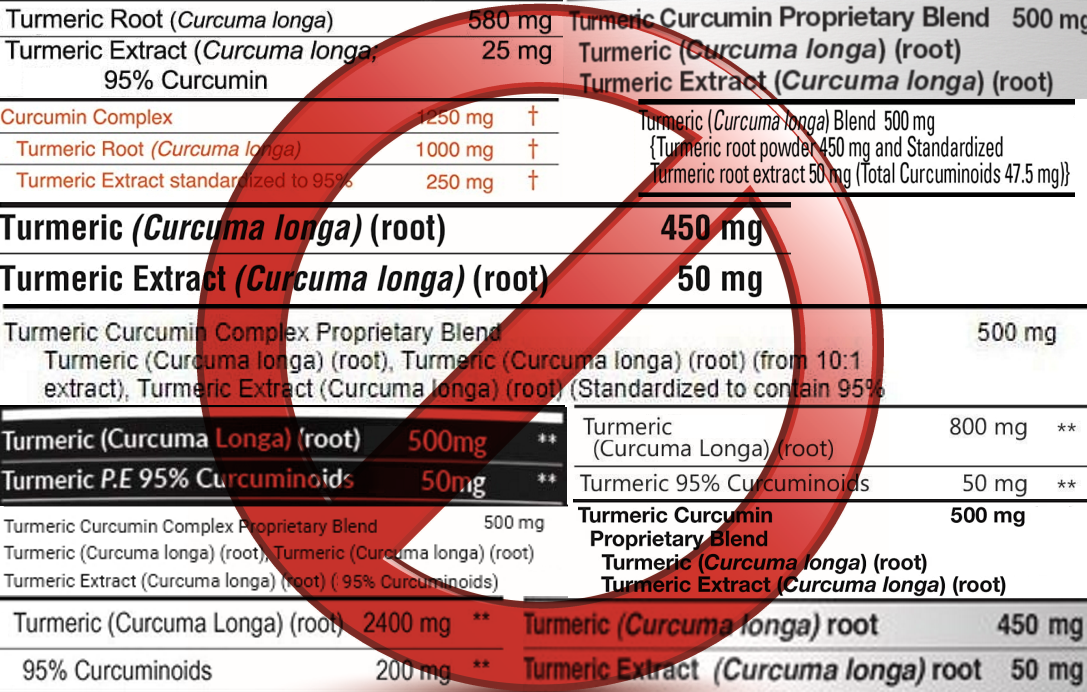
how do other "curcumin" suppliers use deceptive marketing and
misleading advertisements?
|
When selecting a
curcumin extract product, it is important to know the difference
between curcumin and turmeric.
Turmeric
root
contains only 3%
curcumin on average.
Consumers may be misled by deceptive marketing to believe there
is more curcumin per capsule than there actually is.
Unfortunately, these deceptions are not uncommon in the supplement
industry today:
HeartWise Inc., doing business as NatureWise, was hit with a
class action lawsuit for falsely advertising that its
dietary supplements contain “2250 mg Per Day” of curcumin,
when each pill actually only contains 750 mg. Plaintiff
Martha Valentine says the dietary supplement actually
requires three capsules to provide the advertised 2250 mg
dosage. She maintains that she was led to believe each of
the 180 capsules in the container contains 2250mg of the
curcumin supplement after reading and relying on the
product’s label that represented “2250 mg Per Day” of
curcumin and “180 vegetarian capsules.” She says that at the
time of her purchase, she did not know the product required
her to take three capsules to reach the full dosage of 2250
mg of curcumin, and if she had known the truth regarding
NatureWise’s misrepresentations and omissions, she would not
have purchased the product. She maintains that this
representation led her to believe that each of the capsules
contained 2250 mg of the curcumin supplement. However, upon
closer inspection of the bottle, NatureWise reveals that
three capsules must be consumed to provide the 2250 mg per
day serving. NatureWise’s misleading representations and
omissions lead consumers to pay a premium for the
supplements because they falsely believe that they are
receiving three times as much curcumin as they actually
receive in each bottle. “NatureWise intentionally fails to
adequately disclose to consumers that more than one capsule
is required to obtain the labeled dosage amount. Defendant
knew and intended that consumers would purchase, and pay a
premium for, a supplement labeled as having a 2250 mg of
curcumin per day, leading consumers to believe that by
taking 1 capsule per day they would be able to get all the
benefits of consuming a large dosage of curcumin." -
Valentine et al v. HeartWise Inc. d/b/a NatureWise and
HeartWise Wonder Inc., Case No. 20-cv-4302, N.D. Cal.
Sam’s West Inc's Member’s Mark
supplements was hit with a class action lawsuit on March 23, 2023 in a Tennessee federal court
for falsely advertising its turmeric curcumin complex
health supplement. According to the lawsuit filed by
plaintiff Matthew Casella, the product’s
front label representations say it contains 500 mg of
“standardized extract” of “turmeric curcumin complex” and
the Supplement Facts on the back label specify that “95%
Standardized Turmeric (Curcuma longa) Extract” was used.
These representations give consumers the impression the
product contains 95% (475mg) of curcuminoids per serving,
Casella says. “However, lab testing conducted by ConsumerLab.com revealed that instead of the expected 475
mg, the product contains only 9.7 mg of curcuminoids per
serving,” he alleges. The ingredients list states that “CurcuWIN
Turmeric Extract” is the extract used, however a report from
ConsumerLab found that “CurcuWin is only 20% curcuminoids.”
If the product contains CurcuWIN, as listed in the
ingredients, the extract cannot also be “95% Standardized
Turmeric (Curcuma longa) Extract,” as listed in the
supplement facts, Casella reasons. “As a result of the false
and misleading representations, the product is sold at
premium price.” -
Matthew Casella, et al. v. Sam’s West Inc., Case No.
3:23-cv-00102, in the U.S. District Court for the Eastern
District of Tennessee.
"Doctor's Curcumin" on the front of the label,
but the details confirm that each capsule is 100% turmeric
spice.
"Turmeric
Curcumin 500mg Enhanced Formula"
only contains 200mg Curcumin extract, and the remainder is 300mg turmeric.
"1000mg Super Complex Curcumin (25%)" is actually
just 250mg of Curcumin extract and the
remaining 750mg is turmeric.
"Turmeric
Curcumin Proprietary Blend 1000mg" but
contains only 50mg Curcumin 95%
extract per capsule, or 3,000mg per bottle (60 capsules x
50mg) and yet is priced higher than our bottle, which
contains a total of 100,000mg of Curcumin 95% extract.
"Premium Turmeric Curcumin
Complex Plus 1500" contains 150mg of Curcumin and 600mg of turmeric spice
per serving, and the serving size is two capsules, meaning
only 75mg of Curcumin 95% extract per capsule.
Actual labels from
"Curcumin" bottles:
|
|
What are the health properties and pharmacological actions of Curcumin? |
"Over 10,000 research papers and over 1000 review articles have
been published to discuss the molecular basis of curcumin’s
attributed antioxidant, anti-inflammatory, antibacterial,
antiapoptosis, anticancer, and antiaging activities." -
Stem Cells Translational Medicine | 2024
"Regardless of health status, adding curcumin to one’s diet
lowers circulating levels of pro-inflammatory bio-markers and
raises levels of anti-inflammatory mediators. Extensive
investigations have explored the beneficial effects of curcumin
in numerous cancer types, encompassing breast cancer, lung
cancer, cancers affecting the digestive system, as well as
hematological cancers. In addition to its therapeutic
attributes, curcumin has been proposed as a prospective agent in
cancer prevention, primarily attributed to its antioxidant and
immunomodulatory properties. Curcumin has also demonstrated its
potential as an agent in the regulation of obesity and metabolic
processes. In addition, curcumin aids in the management of
metabolic syndrome and hyperlipidemia and has shown promise as a
robust endocrine system modulator, boosting or controlling the
release of several hormones, including insulin. Curcumin has
also exhibited beneficial effects in various pulmonary
disorders, highlighting its potential in the management of
respiratory system-related conditions. Curcumin also exhibits
the capacity to attenuate the natural response of the body to
cutaneous wounds, including inflammation and oxidation. Findings
from a meta-analysis study on human subjects affected by
depression have also revealed that curcumin might improve
depressive and anxiety symptoms in these individuals,suggesting
that it can be utilized as an adjunct treatment for depressive
disorders. The clinical utility of curcumin extends to the realm
of neurodegenerative diseases; dementia, Alzheimer’s disease and
Parkinson's disease. Due to its free radical scavenging,
mitochondrial protecting, anti-inflammatory, and iron-chelating
properties, curcumin is regarded as a promising therapeutic and
nutraceutical agent for the treatment of Parkinson's disease.
Accordingly, several studies have indicated that curcumin
prevents the dopaminergic neuronal loss in models of Parkinson's
disease, which provides further evidence for a potential
neuroprotective role for curcumin in Parkinson's disease." -
Heliyon | 2024
"Studies on curcumin have since
confirmed its powerful antioxidant properties, preventing both
the formation of free radicals and their neutralization, having
anti-inflammatory, antibacterial, immunological, and
neuroprotective properties, as well as being a regulator of the
intestinal microbiota with beneficial effects on the clinical
manifestations of metabolic syndrome." -
Antioxidants |
2024
"Curcumin, the most abundant curcuminoid
in turmeric, has been widely investigated for its various
biological activities, including anti-inflammatory, antioxidant,
and anticancer effects. Numerous in vitro and in vivo studies
have demonstrated the ability of curcumin to modulate multiple
signaling pathways involved in carcinogenesis, leading to
inhibition of cancer cell proliferation, induction of apoptosis,
and suppression of metastasis. Furthermore, curcumin has shown
promising potential as a radioprotective agent by mitigating
radiation-induced oxidative stress and DNA damage. Additionally,
turmeric extracts containing curcuminoids have been reported to
exhibit potent antioxidant activity, scavenging free radicals
and protecting cells from oxidative damage. The multifaceted
pharmacological properties of turmeric make it a promising
candidate for the development of novel therapeutic strategies
for cancer prevention and treatment, as well as for the
management of oxidative stress-related disorders." -
Frontiers in Nutrition | 2024
"Due
to its variety of biological effects and implications in
different diseases, curcumin has been described as “pharmacodynamically
fierce". Curcumin has been found to possess a variety of
biological properties, including its immunomodulatory,
anti-inflammatory, antioxidant, neuroprotective, and anti-cancer
effects. In addition, curcumin has been studied in different
diseases, such as Alzheimer’s disease, pancreatitis, psoriasis,
arthritis, cardiovascular diseases, and cancer. Curcumin has
been reported to possess both preventive and therapeutic effects
on several types of cancer, such as breast and prostate cancer,
lung cancer, pancreatic malignancies, and brain tumors,
including glioblastoma.The anti-cancer properties of curcumin
have been attributed to its diverse interactions with key
molecular pathways implicated in different biological processes,
such as proliferation, control of the cell cycle, apoptosis,
metastasis, angiogenesis, and inflammation. Moreover, curcumin
has been found to specifically target cancer stem cells, a
population of cells largely responsible for the resistance to
established chemotherapeutic treatments and recurrence of cancer
patients. Furthermore, several studies have shown that curcumin
may sensitize tumor cells to both chemo- and radiotherapy and,
thus, improve treatment efficacy while reducing side effects
when given as an adjuvant therapy. As more clinical evidence
becomes available, curcumin is likely to become a fundamental
part of cancer therapies in the future." -
The Role of
Curcumin in Cancer | 2024
"Curcumin taken
orally has been found to counteract cell damage from the
environment and disease." -
Prevention | 2024
"Curcumin, derived
from turmeric, has long captivated the pharmaceutical field
globally due to its pharmacological effects and clinical
development (Hasanzadeh et al., 2020). Curcumin is a lipophilic
polyphenol that is antioxidant, anti-inflammatory, and
anti-fibrotic. It has been extensively used as a food seasoning
because of its high level of safety (Bavarsad et al., 2019)
(Figure 1). Numerous human clinical trials have extensively
employed curcumin for interventions in various diseases, such as
multiple myeloma, pancreatic cancer, NAFLD, colon cancer, and
Alzheimer’s disease, both in vitro and in animal models (Hatcher
et al., 2008)." -
Frontiers in Pharmacology | 2024
"In
the last ten years, there has been an increase in interest in
curcumin-based therapies for the prevention and treatment of
various illnesses, such as cardiovascular diseases, cancers,
neurodegenerative diseases (Parkinson's, Alzheimer's and
multiple sclerosis), autoimmune diseases (osteoarthritis and
rheumatoid arthritis), diabetes, pulmonary diseases and some
gastrointestinal disorders. Curcumin has been shown to have a
positive impact on several inflammatory disorders, including
diabetes and cardiovascular conditions. According to the
available published literature, it can be concluded that
curcumin, as a promising nutraceutical-based treatment not only
has anti-inflammatory, antioxidant, and anti-apoptotic effects,
but it can also have beneficial effects on cardiovascular
diseases by increasing the bioavailability of nitric oxide and
exerting an effect on nitric oxide mediator signals." -
Nitric Oxide | 2024
"Distinguished by its unique molecular structure, curcumin
exhibits potent biological activities including
anti-inflammatory, antioxidant, and potential anticancer
effects. The research points towards curcumin’s growing
importance as a multi-faceted natural compound in the medical
and scientific community. In the realm of contemporary science,
curcumin has sparked considerable interest due to its potential
health benefits. Studies have delved into its effectiveness
against chronic illnesses such as cancer, Alzheimer’s disease,
heart diseases, and inflammatory conditions. This interest is
fueled by its properties as an antioxidant, anti-inflammatory,
and role in cancer prevention. Scientists are examining how
curcumin influences various cellular processes by interacting
with multiple signaling molecules, including growth factors,
cytokines, and the genes involved in cell life cycle and
division. In summary, curcumin, with its deep-rooted history in
traditional healing practices and its promising prospects in
modern medical research, continues to be an area of keen
scientific focus. As research progresses to elucidate the
complexity of its chemistry and the breadth of its
pharmacological actions, curcumin stands as a key player in the
treatment of a diverse spectrum of health conditions." -
International
Journal of Molecular Sciences | 2024
"Curcumin is one of the most powerful natural
anti-inflammatories in existence." -
Curcumin in Alzheimer’s Disease and Depression | 2024
"Intensive studies carried out within the past 3 decades
confirmed that the anti-inflammatory and antitumor properties of
turmeric are attributable to its active component, curcumin.
Curcumin is a natural compound isolated from the rhizome of the
plant Curcuma longa (turmeric) that has been used to
treat inflammation, cancer and neurodegenerative diseases such
as multiple sclerosis (MS) and Parkinson’s disease. A large number of studies including both animal model
experiments and clinical trials, have verified the
anti-inflammatory and immunomodulatory properties of curcumin."
-
Frontiers in Pharmacology | 2024
"Almost 7000 scientific papers on turmeric and almost 20,000
on curcumin have been published in PubMed. These studies
show that the golden spice has enormous health and medicinal
benefits for humans." -
Pharmacology
& Translational Science | 2023 |
|
|
|
First shown to have
anti-bacterial activity in 1949, curcumin has
since been shown to have anti-inflammatory,
anti-oxidant, pro-apoptotic, chemopreventive,
chemotherapeutic, anti-proliferative, wound
healing, anti-nociceptive, anti-parasitic, and
anti-malarial properties as well. Numerous
clinical and preclinical studies and trials
evaluating curcumin's safety and efficacy have
revealed its potential against a wide range of human diseases
and ailments have been completed. These
pathologies include diabetes, obesity, neurologic and
psychiatric disorders, and cancer, as well as
chronic illnesses affecting the eyes, lungs,
liver, kidneys, and gastrointestinal and
cardiovascular systems. Curcumin has
also been shown to regulate numerous transcription
factors, cytokines, protein kinases, adhesion
molecules, redox status and enzymes that have
been linked to inflammation. Growing experimental evidence reveals that
curcumin exhibits multitarget biological
implications signifying its crucial role in
health and disease, with pharmacological effects
against numerous diseases like neuronal,
cardiovascular, metabolic, kidney, endocrine,
skin, respiratory, infectious, gastrointestinal
diseases and cancer. The ability of curcumin to
modulate the functions of multiple signal
transductions are linked with attenuation of
acute and chronic diseases.
Extensive research over the past
half century has shown that curcumin
(diferuloylmethane), a component of the golden
spice turmeric (Curcuma longa), can
modulate multiple cell signaling pathways.
Extensive clinical trials over the past quarter
century have addressed the pharmacokinetics,
safety, and efficacy of this nutraceutical
against numerous diseases in humans. Some
promising effects have been observed in patients
with various pro-inflammatory diseases including
cancer, cardiovascular disease, arthritis,
uveitis, ulcerative proctitis, Crohn’s disease,
ulcerative colitis, irritable bowel disease,
tropical pancreatitis, peptic ulcer, gastric
ulcer, idiopathic orbital inflammatory
pseudotumor, oral lichen planus, gastric
inflammation, vitiligo, psoriasis, acute
coronary syndrome, atherosclerosis, diabetes,
diabetic nephropathy, diabetic microangiopathy,
lupus nephritis, renal conditions, acquired
immunodeficiency syndrome, β-thalassemia,
biliary dyskinesia, Dejerine-Sottas disease,
cholecystitis, and chronic bacterial
prostatitis.
|
|
Curcumin has also shown protection against
hepatic conditions, chronic arsenic exposure, and alcohol intoxication.
Extensive preclinical
studies over the past three decades have indicated curcumin’s
therapeutic potential against a wide range of human diseases. In
addition, curcumin has been shown to directly interact with
numerous signaling molecules. These preclinical studies have
formed a solid basis for evaluating curcumin’s efficacy in
clinical trials. The clinical trials conducted thus far have
indicated the therapeutic potential of curcumin against a wide
range of human diseases. Curcumin has a potential to prevent
and/or manage various diseases due to its anti-inflammatory,
anti-oxidant and anti-apoptotic properties with an excellent
safety profile. In contrast, the anti-cancer effects of curcumin
are reflected due to induction of growth arrest and apoptosis in
various premalignant and malignant cells. Curcumin reduces the
risk of osteoporosis via amelioration of mitochondrial membrane
function, PKB phosphorylation, microRNA-365 activation,
osteoblasts proliferation. It reduced ulcerative colitis by
inhibiting neutrophil chemotaxis. The gastroprotective effect is
due to inhibition of acid release, amelioration of blood flow,
angiogenesis and collagenization of gastric tissue. Curcumin
shows hepatoprotective action due to inhibitory activity against
NF-jB. Additionally, curcumin reduced liver marker enzymes,
cholesterol levels and replication of hepatitis B and C viruses.
Curcumin treatment reduces asthma and allergy symptoms mainly
due to inhibition of histamine release, attenuation of IgE,
inhibition of COX-2 enzyme, suppression of JNK54/56, ERK 42/44
and p38 MAPK, stimulation of Nrf-2/HO-1 pathway, upregulation of
Notch1, Notch2 receptors, GATA3 etc. Curcumin blocks certain
cytokines and enzymes, inhibits ROS generation, downregulate
NF-kB activation, induce extracellular matrix production,
upregulate collagen and fibronectin expressions thereby reduce
inflammatory diseases. Curcumin treatment reduces fibronectin
and collagen IV expressions, suppresses TGF-bsignaling and
exhibits antioxidant, anti-inflammatory and anti-apoptotic
potential thereby ameliorates kidney functions. Studies have indicated the
anticancer effects of curcumin by evaluating its effect on a
variety of biological pathways involved in cell cycle
regulation, apoptosis, tumorigenesis, mutagenesis and
metastasis.
|
Curcumin mediates its effects
by modulation of various molecular targets including
transcription factors, enzymes, cell cycle proteins, receptors,
cell surface adhesion molecules, neurotransmitters etc. Curcumin
exhibits antioxidant, anti-inflammatory and anti-apoptotic
potential thereby reduce neurodegenerative, cardiovascular,
metabolic, gastrointestinal, respiratory and inflammatory
diseases. Clinical and preclinical data have conclusively proved
that curcumin modulates neurotransmitter levels and reduces
neurodegeneration thereby ameliorate neuronal and behavioral
dysfunctions. Curcumin reduces Alzheimer’s pathology by reducing
Abplaques and tau phosphorylation. The anti-depressant and
anxiolytic mechanism of curcumin includes inhibition of brain
MAO activity, modulation of serotonin receptor and amelioration
of neurotrophic factors. Curcumin reduces drug addiction and
withdrawal symptoms, possibly through modulation of HAT, DNA
methyl transferases, CREB, BDNF and CaMKIIalevels. Curcumin
administration reduced Huntington’s disease by reducing
huntingtin aggregates. In cardiovascular disease, the
anti-atherosclerotic mechanism of curcumin includes the
inhibition of platelet aggregation and modulation of cholesterol
homeostasis. Curcumin effectively reduce hypertension by
blocking angiotensin I receptor, reducing circulating
angiotensin-converting enzyme and inducing vasodilation. The
antiarrhythmic mechanisms of curcumin are due to modulation of
Ca 2þ homeostasis and blockade of potassium channels.
The
anti-fungal mechanisms of curcumin includes the leakage of
intracellular component, disruption of plasma membrane,
generation of oxidative stress, induction of apoptosis,
inhibition hyphae development, upregulation of chitin synthase
and PKC etc. Curcumin treatment downregulated genomic
transcription and translation, inhibited viral oncoproteins,
suppressed the Akt/SREBP-1 pathway, inhibited hemagglutination,
proteases, integrase and Tat protein acetylation resulting in
antiviral effects.
Curcumin
administration reduces cerebral infracts size and volume during
stroke. During metabolic diseases, curcumin treatment
ameliorates b-cell dysfunction, insulin signaling and GLP-1
secretion while reduces glucose intolerance, hyperglycemia,
hyperinsulinemia and hyperlipidemia.
|
|
|
| what research papers on
Curcumin studies have been published in medical literature and scientific journals? |
|
October 2024
Curcumin blunts epithelial-mesenchymal transition to
alleviate invasion and metastasis of prostate cancer through the
JARID1D demethylation
Cancer Cell
International | October 2024
Curcumin, fundamentally a
polyphenolic compound, is derived as a powder from the rhizome
of the perennial herb, Curcuma longa. Previous studies
have shown that curcumin, as a multi-target drug, has
anti-inflammatory, antioxidant, anti-infective, anti-fibrotic,
and anti-atherosclerotic properties, which could significantly
inhibit the occurrence and metastasis of various malignant
tumors. In addition, mounting evidence indicates that curcumin
can act as an epigenetic regulator to exert anti-tumor effects,
improve the expression of histone deacetylases, DNA
methyltransferases, and inhibit the invasion and migration of
tumors. The results demonstrated that the invasion and
metastasis of prostate cancer induced by the knockdown of
JARID1D were significantly inhibited by curcumin. Curcumin
inhibits the metastatic potential of CRPC cells by regulating AR
and EMT-related genes through methylation of JARID1D. These
results underscore the therapeutic potential of curcumin in the
treatment of invasive prostate cancer. Curcumin, a promising
anti-cancer agent, has garnered significant interest for its
ability to modulate multiple signaling pathways, thereby
inhibiting the proliferation, migration, and invasion of
prostate cancer cells. Our investigation revealed that curcumin
effectively suppressed the growth of 22RV1 cells at an IC50 of
12.65 µM and enhanced both the expression and demethylation
activity of JARID1D at a 14 µM dose. This suggests a capacity of
curcumin to upregulate JARID1D expression through demethylation,
concurrent with its growth inhibitory effect on prostate cancer
cells. Curcumin can affect the progression of PCa through
the PI3K and Wnt signaling pathways [33, 34], but it is more
related to the growth and proliferation of prostate cancer
cells. In JARID1D mediated invasion and metastasis of PCa, the
PI3K and Wnt signaling pathways do not play a dominant role.
Both previous report and our study confirm that curcumin may has
a DNA demethylation effect on tumor cells. Further experiments
demonstrated that curcumin could significantly inhibit the
proliferation of PCa cells with different phenotypes Treatment
with curcumin significantly inhibited PCa metastasis caused by
JARID1D knockdown and decreased the expression of N-cadherin,
Vimentin and MMP2, but stimulated the expression of JARID1D, AR
and E-cadherin. These results highlight curcumin’s potential to
impede prostate cancer invasion and metastasis, particularly
when JARID1D is compromised, through the regulation of AR/EMT
pathways. In summary, although curcumin has shown certain
anti-cancer effects in vitro and animal models, further
investigation is required to understand its long-term impact and
bioavailability in humans. Furthermore, the administration
strategy of curcumin combined with JARID1D agonist may be more
beneficial to inhibit prostate cancer metastasis. Our study
revealed that the epigenetic modifier JARID1D could restrict the
progression of prostate cancer, and curcumin could stimulate the
expression level of JARID1D and regulate the transcriptional
regulation of AR through demethylation. Taken together, these
findings provide the evidence that curcumin has therapeutic
potential for invasive PCa, and targeting JARID1D may be a
viable therapeutic strategy, particularly in metastatic
prevention.
Curcumin suppresses cell viability in breast cancer
cell line by affecting the expression of miR-15a-5p
Turkish Journal of Biochemistry | September 2024
The
turmeric plant (Curcumin longa) is used to extract
curcumin, a hydrophobic and polyphenolic substance with potent
anti-cancer effects. There has been an increased interest in
research into curcumin’s effect on non-coding RNAs (ncRNA) in
cancer. Some essential cell processes, including proliferation,
differentiation, and migration, can be altered as a result of
epigenetic modifications. ncRNA dysregulation, known to play a
key epigenetic regulatory function in the genome, has been
associated with a variety of diseases, including cancer.
Integrating curcumin and tumor suppressor miRNAs in therapy for
cancer may boost bioavailability and lead to greater success in
the battle against it. Anti-cancer effects of curcumin have been
reported in the literature. The discovery that it can produce
this effect by changing the expressions of protein-coding and
non-coding RNA molecules has paved the way for new research. In
the literature on miR-15a-5p, the effect of which we want to
examine in breast cancer in our study, it is stated that
curcumin-mediated expression of miR-15a-5p in leukemia cells can
be increased, and thus, the expression of the WT1 oncogene can
be controlled. Curcumin provides hope in the battle against
cancers as research continues to uncover new therapies. Curcumin
research is ongoing globally because of its antioxidant and
antiviral effects in addition to anti-cancer capabilities. Due
to these properties, its importance has been emphasized in the
literature not only for cancer but also for many
neurodegenerative and metabolic diseases. Curcumin was taken
into a clinical phase I trial (NCT03980509) and phase II study
(NCT03072992) combined with paclitaxel in BRCA. These clinical
phase studies mainly focused on the effects of curcumin on
disease recurrence and metastasis. A clinical Phase III trial of
curcumin on prostate cancer patients (NCT03769766) is still
ongoing. In addition to the idea of using curcumin in
combination with various chemotherapeutics, it has been
suggested that utilizing it in combination with miRNAs in cancer
could strengthen its anti-cancer role. With the discovery of
curcumin’s ability to modify miRNA expression, an alternative
field for research in cancer-targeted treatment has emerged. In
light of the outcomes of our study, we would like to suggest
that curcumin’s anti-cancer properties could be strengthened
through miR-15a-5p, and a combination of curcumin/miR-15a-5p
studies in breast cancer could be more beneficial for cancer
therapy.
Exploring the Renoprotective Potential of Bioactive
Nutraceuticals in Chronic Kidney Disease Progression: A
Narrative Review
Cureus | September 2024
Curcuminoids, including curcumin, demethoxycurcumin, and
bisdemethoxycurcumin, are the active compounds found in
turmeric. Curcumin's potential mode of action in CKD is by
focusing on its effects on endogenous intestinal alkaline
phosphatase (IAP). IAP is an enzyme found in the intestines that
plays a crucial role in maintaining gut homeostasis and
protecting against inflammation and oxidative stress. Curcumin
stimulates the production and activity of IAP, leading to
beneficial effects in CKD. The activation of IAP by curcumin is
suggested to have multiple protective mechanisms, including the
reduction of oxidative stress, inflammation, and fibrosis in the
kidneys. By enhancing IAP activity, curcumin may help maintain
gut barrier integrity and reduce the translocation of toxins and
harmful substances into the bloodstream, positively impacting
kidney health in CKD patients.
The Effect of Curcumin on Reducing Atherogenic Risks
in Obese Patients with Type 2 Diabetes: A Randomized Controlled
Trial
Nutrients | August 2024
Curcumin,
derived from turmeric root, exhibits notable anti-inflammatory
effects. The curcumin intervention significantly reduced pulse
wave velocity and improved cardiometabolic risk profiles. These
findings suggest that curcumin treatment may effectively reduce
atherogenic risks in type 2 diabetes patients with obesity.
Curcumin (Curcuma longa), the principal component of
the spice turmeric, has recently garnered interest for its
potential benefits in addressing various health conditions,
notably metabolic syndrome. Research has highlighted the
antihypercholesterolemic properties of curcumin extract,
including a reduction in cholesterol and triglyceride (TG)
levels and decreased vulnerability to low-density lipoprotein
cholesterol (LDL-C). The anti-atherosclerotic and protective
effects of curcumin against coronary heart disease have also
been recognized. Additionally, curcumin has notable potential in
managing metabolic diseases due to its anti-inflammatory,
antioxidant, and lipid-modifying properties. It enhances insulin
sensitivity and supports weight management. Curcumin also
protects liver health and offers cardiovascular benefits by
reducing inflammation and oxidative stress. These multifaceted
effects make it a promising complementary therapy for conditions
like obesity, type 2 diabetes, and cardiovascular diseases.
Curcumin, a bioactive compound in turmeric, shows potential for
reducing atherogenic risk by mitigating inflammation and
improving cardiometabolic factors. These effects are promising
for protecting against atherosclerosis and related
cardiometabolic risks in type 2 diabetes patients with obesity.
By reducing cardiometabolic risk factors, curcumin provides
various health benefits, making it a valuable component of a
balanced diet and healthy lifestyle.
Effect and mechanism of curcumin on colon cancer
cell senescence through early growth response 1 (EGR1)
Translational Cancer Research | August 2024
Curcumin
inhibited the transcriptional activity of EGR1, thereby
promoting cell senescence and inhibiting tumor progression.
Curcumin hampers the activity of TF EGR1, affecting the
transcription and translation of target genes TERT and SIRT6,
thus promoting cell senescence and inhibiting colon cancer cell
proliferation. These findings provide potential insights for
targeted therapy of colon cancer. Curcumin exhibits diverse
anti-tumor properties, including the inhibition of tumor cell
proliferation, metastasis, and invasion, rendering it a
promising candidate for the prevention and treatment of multiple
cancer. Curcumin, a traditional Chinese medicine derived
primarily from the rhizome of turmeric, has been identified by
modern pharmacological studies as having multiple benefits for
the human body, including its anti-inflammatory, anti-tumor,
anti-angiogenesis, anti- metastasis, and anti-multidrug
resistance activities. Curcumin, a natural compound derived from
the turmeric plant, has garnered attention for its potential
anticancer properties. Prior investigations have underscored
curcumin’s induction of cell senescence across various cancer
types, including colon cancer. Our research extends these
findings by delineating the mechanism through which curcumin
orchestrates the transcription and expression of key genes such
as TERT and SIRT6, mediated by EGR1, thereby contributing to
cell senescence.The capacity of curcumin to induce cell
senescence bears significant implications for treating colon
cancer. Senescence cells lose their proliferative potential,
thereby impeding tumor growth. Moreover, curcumin has been
reported to curb the metastatic propensity of colon cancer cells
by stifling epithelial-mesenchymal transition (EMT), a pivotal
process in initiating metastasis. Curcumin dampens EGR1 activity
and promotes senescence, consequently hindering tumor
progression.In conclusion, curcumin mitigates the activity of
the TF EGR1, augments the expression of target genes TERT and
SIRT6, and accelerates tumor cell senescence. This positions
curcumin a potential candidate for colon cancer therapy,
offering an alternative approach to clinical treatment. Its
ability to inhibit cell proliferation and metastasis underscores
its therapeutic potential in colon cancer. Therefore, strategies
targeting EGR1 with curcumin may represent a viable option for
untreated colon cancer.
Curcumin Induces Autophagy-mediated Ferroptosis by
Targeting the PI3K/AKT/mTOR Signaling Pathway in Gastric Cancer
Gastroenterology | August 2024
The polyphenol compound
curcumin shows prominent anti-tumor effects in multiple cancer
types, including gastric cancer. Curcumin attenuated cell
viability but stimulated cell death in gastric cancer cells.
Curcumin enhanced autophagy in gastric cancer cells, as
demonstrated by the increased levels of ATG5, ATG7, Beclin 1,
and LC3B. Besides, curcumin upregulated iron, MDA, GSH, and
ACSL4 levels while downregulated lipid ROS, SLC7A11, and GPX4
levels, suggesting its stimulation on ferroptosis in gastric
cancer cells. Curcumin decreased p-PI3K, p-AKT, and p-mTOR
levels in cells. Importantly, the ferroptosis inhibitor
ferrostatin-1 overturned the impacts of curcumin on gastric
cancercell viability, death, and ferroptosis. Curcumin
suppresses gastric cancer development by inducing
autophagy-mediated ferroptosis by inactivating the PI3K/AKT/mTOR
signaling. Curcumin has been confirmed to possess anti-oxidant,
anticancer, anti-inflammatory, anti-analgesic, lipid-modifier,
and anti-microbial effects. Curcumin has previously been
reported to suppress tumorigenesis in many types of human
cancers by facilitating ferroptosis. In summary, our research
confirms that curcumin sup- pressed cell growth but promotes
cell death in gastric cancer cells by stimulating ferroptosis.
Mechanistically, curcumin induces autophagy and inactivates the
PI3K/AKT/mTOR pathway in gastric cancer cells. Hence, curcumin
might suppress gastric cancer development by inducing
autophagy-mediated ferroptosis by restraining the PI3K/AKT/mTOR
signaling. Our observations might expand our knowledge of the
effects of curcumin on ferroptosis in gastric cancer and provide
a novel direction for the potential use of curcumin in gastric
cancer treatment.
Curcumin for
COVID-19: 27 studies from 240 scientists and 14,886 patients in
11 countries
C19early.org | August 2024
Curcumin reduces risk for COVID-19 with very high confidence for
mortality, hospitalization, recovery, and in pooled analysis,
high confidence for viral clearance. Statistically significant
improvements are seen for mortality, hospitalization,
progression, recovery, and viral clearance. 18 studies from 16
independent teams in 8 countries show statistically significant
improvements. Meta analysis using the most serious outcome
reported shows 44% lower risk. Results are similar for
Randomized Controlled Trials, higher quality studies, and
peer-reviewed studies. Other meta analyses for
curcumin can be found showing significant improvements for
mortality, hospitalization, recovery, and symptoms.
Evaluation of the effectiveness of curcumin and
piperine co-supplementation on inflammatory factors, cardiac
biomarkers, atrial fibrillation, and clinical outcomes after
coronary artery bypass graft surgery
Clinical Nutrition ESPEN | August 2024
Supplementation with
curcumin-piperine had a promising effect on serum CRP and TAC.
It also had a favorable impact on CK-MB among patients who
underwent CABG surgery.
The
Bright Side of Curcumin: A Narrative Review of Its Therapeutic
Potential in Cancer Management
Cancers |
July 2024
Curcumin, a polyphenolic compound derived from
Curcuma longa, exhibits significant therapeutic potential in
cancer management. Curcumin demonstrates strong antioxidant and
anti-inflammatory properties, contributing to its ability to
neutralize free radicals and inhibit inflammatory mediators. Its
anticancer effects are mediated by inducing apoptosis,
inhibiting cell proliferation, and interfering with tumor growth
pathways in various colon, pancreatic, and breast cancers.
Curcumin has been used for health maintenance and disease
management, dating back to ancient Indian and Chinese medicine
over 4000 years ago. In these traditional practices, turmeric,
containing curcumin, was used to treat various conditions such
as respiratory disorders, liver diseases, anorexia, rheumatism,
and diabetic wounds. Curcumin, as highlighted in the literature,
holds immense promise in health. Its antioxidant properties
enable it to neutralize free radicals and protect cells from
oxidative damage, while its anti-inflammatory action inhibits
the expression of inflammatory mediators and reduces
inflammation at the cellular level. Curcumin has demonstrated
neuroprotective effects and potential benefits in treating
neurodegenerative diseases. Recent studies have underscored the
role of curcumin in reducing brain inflammation, protecting
neurons, and promoting neurogenesis. Moreover, numerous studies
have illuminated the anticarcinogenic potential of curcumin in
various types of cancer, including colon, pancreatic, and other
high-risk cancers. Curcumin has been studied for its ability to
inhibit tumor cell growth, induce apoptosis, and reduce
resistance to chemotherapy and radiotherapy. Curcumin may
reverse cancer progression through the inhibition of
IL-6R/STAT3. It also sensitizes cancer to antitumor and
antimetastatic effects by suppressing the NF-kappa B cell
signaling pathway. Therefore, curcumin is an attractive
candidate for combination chemotherapy due to its broad spectrum
of biological activities. Its anti-inflammatory, antioxidant,
and antitumor activities improve the efficacy of conventional
chemotherapeutic drugs while reducing side effects. Curcumin may
reverse cancer progression through the inhibition of
IL-6R/STAT3. It also sensitizes cancer to antitumor and
antimetastatic effects by suppressing the NF-kappa B cell
signaling pathway. Therefore, curcumin is an attractive
candidate for combination chemotherapy due to its broad spectrum
of biological activities. Its anti-inflammatory, antioxidant,
and antitumor activities improve the efficacy of conventional
chemotherapeutic drugs while reducing side effects. In
colorectal cancer, curcumin has been shown in vitro to sensitize
cancer cells to 5-fluorouracil (5-FU) and oxaliplatin. Studies
have shown that curcumin enhances the cytotoxic effects of these
drugs by modulating several signaling pathways, including NF-κB
and STAT3, leading to increased apoptosis and the inhibition of
cell proliferation, migration, and invasion. A synergistic
effect in overcoming drug resistance was demonstrated by Howells
et al., who found that curcumin enhanced resistance to
oxaliplatin chemo induction in both in vitro and in vivo colon
cancer cells. Tian et al. showed that curcumin potentiates the
antitumor effects of 5-FU in esophageal squamous cell carcinoma
cells by downregulating NF-kappa B signaling, leading to
increased apoptosis both in vitro and in vivo. Hartojo et al.
reported complementary mechanisms of apoptotic pathway
activation, stating that the combination of curcumin and
cisplatin had an additive effect in inducing apoptosis in
esophageal adenocarcinoma cells. Curcumin showed synergistic
effects with paclitaxel and doxorubicin in breast cancer cell
lines. Farghadani and Naidu, Mohammadian et al., and Vinod et
al. found that the combination of curcumin and these
chemotherapeutic agents downregulated HER2 and EGFR, which are
often overexpressed in breast cancer, inhibited cell
proliferation, and induced apoptosis. Curcumin sensitizes breast
cancer cells to 5-FU by modulating signaling events involved in
cell survival and apoptosis. In clinical trials, patients with
advanced breast cancer who received curcumin combined with
docetaxel showed higher response rates compared to docetaxel
alone. The combination was well tolerated and resulted in fewer
side effects. This suggests the potential of curcumin to improve
the efficacy of chemotherapy. The combination of curcumin and
gemcitabine significantly reduced tumor growth compared to
either agent alone in a mouse model of pancreatic cancer. The
combination therapy worked by inhibiting angiogenesis and
inducing apoptosis by suppressing the NF-κB and COX-2 pathways.
Yoshida et al. showed that curcumin can sensitize human
pancreatic cancer cells to gemcitabine, potentially overcoming
gemcitabine resistance. Treatment with a combination of curcumin
and cisplatin significantly reduced tumor size and weight in a
mouse xenograft model of ovarian cancer. The combination therapy
was more effective than either agent alone. Also, it attenuated
cisplatin-induced nephrotoxicity, highlighting curcumin’s
protective role against chemotherapy’s side effects. A phase II
clinical trial in patients with metastatic colorectal cancer
showed that adding curcumin to the standard FOLFOX regimen
(5-FU, leucovorin, and oxaliplatin) improved overall survival
and progression-free survival compared to the standard regimen
alone. Therefore, combining curcumin with chemotherapeutic
agents in vitro and in vivo has shown a synergistic effect in
cancer therapy. In summary, curcumin demonstrates substantial
therapeutic potential in cancer treatment due to its
multifaceted biological activities, including antioxidant,
anti-inflammatory, and anticancer properties, exerting its
potential therapeutic effects against various types of cancer.
For example, curcumin exhibits substantial anticancer properties
particularly against gastrointestinal cancers, such as
colorectal and pancreatic cancer, by inducing apoptosis,
inhibiting cell proliferation, and modulating multiple cell
signaling pathways. Additionally, studies have shown promising
results in the treatment of breast cancer, where curcumin
interferes with cancer cell growth and metastasis. The compound
has also demonstrated efficacy in prostate cancer by targeting
androgen receptor signaling and in head and neck cancers through
the inhibition of tumor growth and angiogenesis
Possible use of Curcuma longa extract as a
post-chemotherapeutic supplement in acute myeloblastic leukaemia
International Journal of Clinical and Diagnostic Pathology |
July 2024
Curcumin, a polyphenolic compound from Curcuma
longa, is well known for its anti-inflammatory,
antioxidant, and anticancer properties. These findings suggest
that curcumin possesses potent cytotoxic effects on leukemia
cells and could serve as a valuable adjunct in leukemia therapy.
Further research, including clinical trials, is necessary to
evaluate the safety and efficacy of curcumin in leukemia
patients and to explore potential synergistic effects with
existing chemotherapy agents. This study delineates the
potential of curcumin as a less toxic and more effective
therapeutic option for leukemia. In conclusion, our research
aligns with previous findings and offers a detailed analysis of
curcumin's significant anti- AML properties. Curcumin's capacity
to trigger apoptosis, suppress cell proliferation, and hinder
the spread of AML cells underscores its potential as a
therapeutic agent. This study highlights the promise of curcumin
as a potent weapon in the battle against acute myeloid leukemia.
Accelerate muscle recovery with curcumin? This is
how you do it
Ergo-log.com | July 2024
Supplementation with curcumin accelerates recovery from
exercise-induced muscle damage, this web magazine has reported
several times. A Chinese meta-study, published in PLoS One,
tells athletes how to best use curcumin Researchers from Huaqiao
University traced 14 previously published trials in the
scientific literature, in which researchers had subjects
exercise intensively - in most cases this involved strength
training - and determined the effect of curcumin supplementation
on muscle recovery. The researchers looked at, among other
things, the effect on muscle soreness. The figure below shows
that supplementation with curcumin significantly reduced muscle
pain.
Curcumin
protects against cadmium-induced germ cell death in the testis
of rats
Toxicology Research | July 2024
Overall, our data suggest that cadmium induces germ cell
apoptosis through mitochondrial-induced oxidative stress, but
curcumin pretreatment offers strong protection against
cadmium-induced reproductive toxicity.
Common spice could help fight off cancer
and reduce signs of ageing
Express | July
2024
Curcumin. It has such powerful anti-inflammatory effects
that some studies even suggest the effects of curcumin are
comparable to those of some pharmaceutical drugs. Curcumin has
also been shown to increase the body's antioxidant capacity.
Oxidative damage and free radicals are believed to be one of the
mechanisms behind accelerating signs of aging and several
chronic diseases. Curcumin, a key polyphenol found in food,
plays a crucial role in lowering oxidative stress and balancing
different bodily functions. It has the ability to decrease
intracellular lipid peroxidation and enhance the body's
antioxidant capabilities for a longer lifespan. Other studies
have also demonstrated that taking curcumin supplements can help
protect the hippocampus, which is responsible for learning and
memory functions in the brain.Curcumin, the active ingredient in
turmeric, may also kill isolated cancer cells in a test tube and
prevent the growth of new blood vessels in tumors, limiting
their ability to spread. Golden milk may be good for your brain,
too. Studies show that curcumin may increase levels of
brain-derived neurotrophic factor (BDNF). BDNF is a compound
that helps your brain form new connections and promotes the
growth of brain cells. Curcumin may help reduce symptoms of
depression, too. Adding in a few grinds of black pepper will
unlock extra health benefits that would otherwise be lost. The
common table seasoning contains piperine - a natural substance
that enhances the absorption of curcumin by 2,000%.
Meta-analysis of the effect of curcumin
supplementation on skeletal muscle damage status
PLOS One | July 2024
Curcumin supplementation
significantly mitigates skeletal muscle damage, with notable
improvements in CK levels, muscle soreness, IL-6 levels, and
ROM. Curcumin’s potential impact on chronic inflammatory
conditions like metabolic syndrome, arthritis, and cancer has
been thoroughly researched in recent decades. Curcumin is
renowned for its myriad health benefits, including antioxidant,
anti-inflammatory, and analgesic effects, which make it a highly
popular supplement. Extracted from turmeric and approved for use
as a food additive in China, curcumin activates SIRT1,
potentially reducing inflammatory responses and protecting
cardiovascular health. It also inhibits COX-2 and NF-kB
signaling pathways while enhancing adaptability to training,
offering similar benefits to NSAIDs without their side effects.
Its effectiveness in alleviating post-exercise inflammation has
contributed to its growing popularity. The use of curcumin
supplements, known for their anti-inflammatory properties, can
reduce inflammation and relieve pain. Curcumin is known to
potentially modulate the inflammatory response following muscle
injury through its effects on the inflammatory cytokine
interleukin-6 (IL-6). This cytokine plays a vital role in both
muscle injury and repair processes. Supplementation with
curcumin could potentially improve muscle function and joint
flexibility. curcumin’s potent antioxidant properties enable it
to neutralize ROS generated within the oxidative phosphorylation
chain. These combined mechanisms contribute to curcumin’s
effectiveness in reducing CK levels. Curcumin alleviates muscle
soreness through multiple mechanisms, notably by inhibiting
COX-2 expression, which reduces CK activity and prostaglandin
release, thereby easing soreness. Meta-analyses have shown
curcumin’s efficacy in reducing post-exercise muscle soreness.
Whether taken before or after exercise, curcumin consistently
reduces muscle soreness.
The effectiveness of turmeric supplementation in
reducing low-density lipoprotein cholesterol in adults
Department of Healthcare and Behavioral Sciences, Medical
Writing Certificate Program, University of California, San Diego
Extended Studies, La Jolla, California, USA | July 2024
Curcumin has potential as a safe, inexpensive dietary supplement
to support cardiovascular health. One study reported curcumin
reduced LDL-C by a mean of 39.83 mg/dL. Panahi et al. provide a
detailed review of the metabolic and cellular MoA of curcumin
and statins, and point out that they both target the same
specific nuclear receptors and enzymes while reducing LDL-C. The
authors note that while statins are more efficient in lowering
LDL-C, for some patients, they have adverse effects. The authors
suggest that for such patients, a combination of a statin and
curcumin could synergistically lower LDL-C to a desirable level,
while requiring a lower dosage of the statin, which could reduce
the risk of adverse effects, such as extreme muscle pain.
Integrating Network Pharmacology, Transcriptomics to
Reveal Neuroprotective of Curcumin Activate PI3K / AKT Pathway
in Parkinson’s Disease
Drug Design,
Development and Therapy | July 2024
Curcumin restored the
dyskinesia and dopaminergic neurons damage of MPTP-induced mice.
Curcumin against Parkinson’s disease by regulating inflammation,
oxidative stress, and aging. The mechanisms of these were
associated with activation of PI3K / AKT pathway. In
conclusion, the neuroprotective mechanisms of curcumin activate
PI3K / AKT pathway in Parkinson’s disease was revealed by our
study. Recently, there has been increasing interest in using
curcumin to prevent Parkinson’s disease. The mechanisms of
curcumin to anti-Parkinson’s Disease may include antioxidant,
Immune modulation, and the clearance of α-syn. Our previous
study suggested that curcumin’s effectiveness in treating
Parkinson’s disease was linked to the gut-brain axis. Recent
reviews have increasingly suggested that curcumin targeting the
PI3K/AKT signal pathway to protect dopaminergic neurons, while
also inhibiting the activation of microglia. As a result,
curcumin has shown promising potential in the treatment and
prevention of Parkinson’s Disease. Either as antioxidants or
modulators of cell signalling, the influence of curcumin on
oxidative and inflammation balance are key. Consistent with our
enrichment analysis results, the neuroprotective of curcumin on
Parkinson’s disease depend on oxidative stress, inflammation and
apoptosis of nerve cells has been demonstrated. However, there
is no direct experimental evidence in vivo for curcumin exerts
the effects of anti-oxidative stress, anti-inflammation and
anti-apoptosis by activating the PI3K/AKT in Parkinson’s
Disease. And AKT gene expression in the cell was the key role of
anti-oxidative stress, anti-inflammation and anti-apoptosis.
Finally, our study verified the PI3K/AKT pathway activated by
curcumin in Parkinson’s disease. However, to further demonstrate
curcumin protects through the activation of the PI3K/AKT
pathway, our future study should be incorporated an inhibitor
targeting the PI3K/AKT pathway. This has established a solid
theoretical basis for the clinical application of curcumin in
treating Parkinson’s disease.
Potential of Curcumin to Reduce Serum Nuclear
Factor-Kappa B (NF-kB) Levels After High-Intensity Exercise
Retos Journal | July 2024
The group given curcumin after
high-intensity exercise reduced serum NF-kB levels significantly
(*p<0.05) compared to the placebo group. It can be concluded
that the administration of curcumin at 400 mg after
high-intensity exercise can reduce serum NF-kB levels. Curcumin
is known for its active compounds that have anti-inflammatory
activity (Boarescu et al. 2022). Curcumin is able to inhibit
inflammation by modulating NF-κB signals and blocking TNF- α
signals by activating protein responses in muscles (Srivastava
et al. 2017; Venkata et al. 2012). The anti-inflammatory
activity of curcumin also inhibits the production of
pro-inflammatory eicosanoids which include prostaglandins and
leukotrienes (Han, Zhang, and Li 2021; Petrone-Garcia et al.
2021; Zhu et al. 2019). Curcumin has been widely used to
increase endurance and Maximal oxygen uptake VO2 max (Hamidie,
Ali, and Masuda 2017). In addition, curcumin has been widely
used in the medical world to accelerate wound healing (Sharma et
al. 2018). Curcumin administered at a dose of 400 mg/day can
reduce serum NF-kB levels. Based on the laboratory tests we
conducted, we believe the reduction in pain intensity occurred
due to the anti-inflammatory effect of curcumin which is able to
modulate NF-kB signals and block TNF-a signals. Reducing
NF-kB levels is believed to lessen pro-inflammatory cytokines
such as TNF-a and is closely related to muscle pain after
exercise. We recommend using curcumin as a natural ingredient
that can potentially reduce NF-kB.
Role of curcumin on beta-amyloid protein, tau
protein, and biochemical and oxidative changes in
streptozotocin-induced diabetic rats
Naunyn-Schmiedeberg's Archives of Pharmacology | July 2024
Results show that curcumin has an effect on reducing oxidative
stress caused by diabetes and increasing antioxidant activity.
The protective effect of curcumin is tested during induction and
active diabetes. The results indicated that diabetic rats
displayed increased levels of Aβ, tau protein, and total oxidant
capacity (TOS) compared to the curcumin-treated groups.
Additionally, the total antioxidant capacity (TAS) levels were
lower in the diabetic rats (P < 0.05). Aβ protein levels are
lower in both the serum and brain of rats with active diabetes
and treated with curcumin compared to control rats (P > 0.05).
In addition, serum TAS levels were higher in rats treated with
curcumin following the induction of diabetes than pre-induction
of diabetes (P > 0.05). The TOS levels in the serum were higher
in the rats treated with curcumin during active diabetes
compared to the rats treated prior to the induction of diabetes.
Curcumin as a regulator of Th17 cells: Unveiling the
mechanisms
Food Chemistry: Molecular
Sciences | July 2024
Curcumin possesses diverse
pharmacological effects due to its interactions with various
cells and molecules. Curcumin can inhibit Th17 proliferation and
reduce the production of inflammatory cytokines. Curcumin, a
polyphenol natural product derived from turmeric, possesses
diverse pharmacological effects due to its interactions with
various cells and molecules. Recent studies have highlighted its
immunomodulatory properties, including its impact on immune
cells and mediators involved in immune responses. Curcumin
exhibits anticancer, anti-inflammatory, and antioxidant
characteristics (Chatterjee and Pandey, 2011, Marjaneh et al.,
2018, Zahedi et al., 2023, Mohajeri and Sahebkar, 2018). In the
past two decades, studies have found that curcumin has
immunomodulatory effects, regulating the activity of immune
cells. It can reduce the production and release of several
proinflammatory cytokines, such as IL-12, TNF, and IL-6, by
preventing the transcription of NF-κB (Jagetia,
2007). Recent reports have shown that curcumin can reduce the
proliferation of alloreactive T cells and significantly suppress
the differentiation of Th17 cells (Park et al., 2013). Curcumin
inhibits the differentiation and development of Th17 cells
through the downregulation of ORγt signaling, IL-21, and IL-6,
as well as the inhibition of STAT3 phosphorylation. In summary,
curcumin has the ability to hinder the differentiation and
proliferation of Th17 cells by repressing the secretion of
IL-23, IL-6, and IL-21. It also suppresses the production of
pro-inflammatory cytokines, including IL-17 and TNF-α, in Th17
cells, thereby reducing the expression of inflammatory cells,
particularly neutrophils, and resulting in decreased
inflammation and infiltration (Haftcheshmeh et al.,
2021). Curcumin demonstrates promise in regulating T cell
subsets associated with inflammatory diseases. Xiao et al. (Xiao
et al., 2022) reported that curcumin downregulated
pro-inflammatory Th17 cells (CD4 + CCR6 + Th17, CD4 + IL-17A +
Th17, IL-17A, BATF, C-Maf, RORγt) and upregulated
anti-inflammatory Treg cells (CD4 + Foxp3 + Treg, CD4 + IL-10 +
Treg, IL-10, Foxp3, Eomes) in mice with diabetic colitis,
suggesting its ability to restore Th17/Treg balance. These
findings highlight the potential of curcumin as a therapeutic
agent for various inflammatory conditions by modulating T cell
responses. Curcumin has been shown to significantly inhibit the
proliferation of Th17 cells and reduce the production of
inflammatory cytokines, including TNF-α, IL-22, and IL-17.
Curcumin has several roles in the regulation of enzymes involved
in inflammation, adhesion molecules, transcription factors,
protein kinases, cytokines, and redox balance. It possesses
various pharmacological functions, including antimicrobial,
antitumor, anti-inflammatory, antiviral, antioxidant, and
antifungal properties (Howells et al., 2021, Heidari et al.,
2023, Bagheri et al., 2020, Cicero et al., 2020, Keihanian et
al., 2018, Khayatan et al., 2022, Mokhtari-Zaer et al., 2018,
Panahi et al., 2019, Sahebkar, 2010, Sahebkar, 2014, Iranshahi
et al., 2010, Mohammadi et al., 2019, Panahi et al., 2012). It
also plays a protective role against autoimmune diseases.
Curcumin, an active compound found in turmeric, exhibits potent
immunomodulatory effects, making it a promising therapeutic
agent for various inflammatory and autoimmune disorders. Recent
studies have focused on the ability of curcumin to modulate
Th17-mediated immune responses, which play a critical role in
the development of several autoimmune diseases. Curcumin has
been shown to inhibit the proliferation and differentiation of
Th17 cells and reduce the production of specific
pro-inflammatory cytokines such as IL-17, IL-6, and TNF-α.
Additionally, curcumin has been found to increase the production
of Treg cells, which suppress excessive immune responses and
maintain immune homeostasis. Overall, curcumin's ability to
target the Th17 axis opens exciting avenues for developing novel
therapeutic strategies for autoimmune and inflammatory diseases.
Ameliorative Effects of Curcumin on Type 2 Diabetes Mellitus
Moleculres | July 2024
Curcumin, the
major curcuminoid of turmeric, is one of the most studied
bioactive components of herbal supplements, which has a variety
of biological activities. Clinical trials and preclinical
research have recently produced compelling data to demonstrate
the crucial functions of curcumin against type 2 diabetes
mellitus via several routes. Curcumin, a natural polyphenol
derived from the rhizome of Curcuma longa (turmeric),
which has been widely used in cosmetics, food, and
pharmaceutical industries, has gained growing interest in the
last years for its pharmacological activities. Different studies
demonstrated that curcumin has anti-oxidant, anti-inflammatory,
antimicrobial, anti-atherosclerotic, nephroprotective,
anticancer, hepatoprotective, immunomodulatory, antidiabetic,
and antirheumatic effects, but with no toxicity. Numerous
studies demonstrated that curcumin could improve insulin
resistance, regulate blood lipid metabolism, decrease glucose
and insulin levels, reduce the release of inflammatory factors,
inhibit oxidative stress, and regulate gut microbiota in
patients with type 2 diabetes mellitus. Results showed that
effectiveness of curcumin on type 2 diabetes mellitus is due to
it being anti-inflammatory, anti-oxidant, anti-hyperglycemic,
anti-apoptotic, anti-hyperlipidemia and other activities.
Numerous studies, including animal and clinical studies, have
provided strong evidence to support curcumin’s crucial role in
type 2 diabetes mellitus prevention due to its
anti-inflammatory, anti-oxidant, antihyperglycemic,
anti-apoptotic, antihyperlipidemic, and other actions. Curcumin
interacts with a number of biomolecules, such as proteins,
nucleic acids, membranes, through non-covalent and covalent
binding. As stated above, curcumin exhibits good hypoglycemic
action and is well taken at high dosages without adverse
effects. It is therefore a potential approach for type 2
diabetes mellitus treatment or prevention. In light of these
results, curcumin may be a promising prevention/treatment choice
for type 2 diabetes mellitus.
Exploring the therapeutic mechanism of curcumin in
prostate cancer using network pharmacology and molecular docking
Helyion | July 2024
Curcumin, a phenolic compound extracted
from turmeric rhizomes, exhibits antitumour effects in
preclinical models of tumours. We identified 307 key targets of
curcumin in cancer treatment. Molecular docking experiments
showed that the binding energies of curcumin to these core
targets were all below −1.85 kJ mol−1, which fully demonstrated
that curcumin could spontaneously bind to these core targets.
Through in-depth network pharmacology and molecular docking
studies, we have found that curcumin may have anticancer
potential by upregulating the expression of PIK3R1 and STAT3,
and downregulating the binding ability of molecules such as SRC,
AKT1, HSP90AA1, ESR1, EGFR, HSP90AB1, MAPK8, and MAPK1. In
addition, curcumin may interfere with the cyclic process of
prostate cancer cells by inhibiting key signalling pathways such
as the PI3K-Akt signalling pathway, MAPK signalling pathway, and
Ras, thereby inhibiting their growth. This study not only
reveals the potential molecular mechanism of curcumin in the
treatment of prostate cancer but also provides an important
theoretical basis for subsequent research. Curcumin exhibits
multifaceted effects in prostate cancer treatment, including
enhancing the sensitivity of prostate cancer to radiotherapy and
chemotherapy, inhibiting cell proliferation, inducing cell
death, inhibiting the expression of the PSA gene, decreasing the
expression of androgen receptor, and decreasing the motility of
tumour cells. In the present study, we systematically elucidated
the possible mechanisms of action of curcumin in prostate cancer
treatment by integrating network pharmacology and molecular
docking. Curcumin acts on multiple targets and pathways involved
in the treatment of prostate cancer. Given that this study
was conducted using relevant databases and the conclusions
lacked experimental support, subsequent biological experiments,
and evidence-based drug validation are required to ensure the
reliability of the findings. In addition, we explored the
molecular mechanism of action of curcumin in treating prostate
cancer, providing a theoretical basis for the future development
and clinical application of this traditional Chinese medicine.
Relationship of Curcumin with Aging and Alzheimer
and Parkinson Disease, the Most Prevalent Age-Related
Neurodegenerative Diseases: A Narrative Review
Nutrition Reviews | June 2024
One of the phytochemicals with
diverse biological properties encompassing antioxidant,
anti-inflammatory, antibacterial, antiviral, anticancer,
antifungal, antidepressant, anti-allergic, and anti-aging
properties is curcumin. Curcumin, a polyphenolic structure with
a distinct orange hue and unique chemical properties, is derived
from the roots of Curcuma longa, a member of the Zingiberaceae
family, commonly known as turmeric. It has been noted that the
incidence of neurodegenerative diseases is low in societies that
consume curcumin widely.
Spices
can boost energy levels and brain function
New York Post | June 2024
Turmeric is derived from the root of the Curcuma longa
plant, a type of ginger. This root contains curcumin, a compound
rich in anti-inflammatory and antioxidant properties. Known as
the “gold” of the plant kingdom, turmeric also supports liver
detoxification and serves as a natural pain killer. By reducing
inflammation and oxidative damage, curcumin shields the brain
from stressors that lead to cognitive decline. “Curcumin boosts
levels of the brain hormone BDNF, which increases the growth of
new neurons and fights various degenerative processes in the
brain,” Crawford explained. In addition, and as The Post
reported, turmeric has been shown to ease symptoms of
indigestion, lower the risk of heart disease, fight depression,
prevent cancer, improve memory, and lessen the pain from
arthritis and other conditions.
Protective role of curcumin in high glucose-induced
osteoblast dysfunctions via Nrf2 activation and ROS/JNK
signaling inhibition
Authorea | June 2024
Curcumin is a polyphenolic phytochemical derived from the
curcuma longa, and it exhibits anti-inflammatory and antioxidant
properties, which associated with cell metabolism. The results
showed that curcumin enhanced the osteoblast differentiation
which are reduced by HG condition via Nrf2 activation and
ROS/JNK inhibition. Knock-down of Nrf2 partially blocked
curcumin-induced Nrf2 activation and ROS/JNK inhibition, as well
as the attenuation of curcumin-induced osteoblast
differentiation and survival in HG condition. JNK inhibition
impair the positive effect of curcumin in response to HG, which
presented a similar result to Nrf2 knock-down. Conclusion:
ROS-related JNK phosphorylation might serve as the mechanism for
HG-induced apoptosis and Nrf2 activation mediated by curcumin
exerted a protective role against osteoblast apoptosis.
Effect of curcumin on gene expression of selected
inflammatory markers in cortex and hippocampus of pilocarpine-induced
seizure in rats on a routine exercise program
Comparative Clinical Pathology | June 2024
The combination of
treadmill exercise program and curcumin administration has been
shown to offer neuroprotection against epileptic seizure but
without detailed mechanism of action being elucidated. This
study therefore evaluated the responses of pro-inflammatory
(tumor necrosis factor alpha [TNFα] and interleukin 6 [IL-6])
and anti-inflammatory (interleukin 10 [IL-10]) cytokines, as
well as the transcription factor regulator (nuclear factor kappa
B [NFκB]) gene expressions to combinatory therapy of curcumin
and treadmill exercise in cortex and hippocampus of pilocarpine-induced
epileptic seizure in rats. Treatment with either curcumin or
curcumin plus exercise significantly ameliorated these
impairments in the inflammatory markers ‘expression levels.
Nevertheless, the combinatory therapy of curcumin and exercise
showed significantly higher ameliorative effects in the cortical
and hippocampal TNFα and IL-10, as well as cortical NFκB and
IL-6. These superior therapeutic properties of the combination
of curcumin and treadmill exercise further support the
therapeutic potentials of this combinatory regimen in the
management of epileptic seizures.
Emerging Anti-Inflammatory Attributes of Curcumin: A Novel
Paradigm and Ameliorative Attributes for the Treatment of
Osteoarthritis
Current Rheumatology Reviews
| June 2024
Strong oxidant, curcumin, is diferuloyl methane;
a member of the class of phenols known as curcuminoids that give
Indian medicinal plants their characteristic turmeric-yellow
hue. Over 5000 years ago, curcumin was first employed in the
traditional Indian medical system. A growing amount of
investigation reveals that curcumin has several pharmacological
characteristics, including anticancer, hepatoprotective,
anti-inflammatory, antioxidant, and antibacterial properties.
Curcumin has been scientifically demonstrated to exhibit
medicinal benefits for osteoarthritis (OA), and further research
is being conducted on the numerous ways through which it
suppresses inflammation and slows the progression of ailments.
Clinical and preclinical studies suggest the potential efficacy
of curcumin in managing osteoarthritis, warranting further
investigation.
Mechanism of 5-fluorouracil induced resistance and role of
piperine and curcumin as chemo-sensitizers in colon cancer
Naunyn-Schmiedeberg's Archives of Pharmacology
| June 2024
Curcumin, a potent phytocompound derived from
Curcuma longa, functions as a nuclear factor (NF)-κB
inhibitor and sensitizer to numerous chemotherapeutic drugs.
Piperine, an alkaloid found in Piper longum, inhibits
cancer cell growth, causing cell cycle arrest and apoptosis.
This review explores the mechanism of 5-FU-induced
chemoresistance in colon cancer cells and the role of curcumin
and piperine in enhancing the sensitivity of 5-FU-based
chemotherapy.
Effects of curcumin in patients with non-alcoholic
fatty liver disease: A systematic review and meta-analysis
Canadian Liver Journal | June 2024
Not only is curcumin
vastly used in food as a spice, but it also has many therapeutic
benefits, as it acts as an anti-inflammatory, antioxidant,
anti-diabetic, anti-hyperlipidemia, immune-modulatory, reno-protective,
anti-cancer, hepato-protective, hypoglycemic, antimicrobial, and
anti-fibrotic. Curcumin treats NAFLD non-alcoholic fatty liver
disease through a series of mechanisms. Firstly, it clears the
liver cells from fat and decreases the synthesis of
triglycerides by inhibiting the enzyme HMG COA reductase.
Secondly, it increases the activation of
cholesterol-7alpha-hydroxylase as well as reduces the absorption
of cholesterol from the intestines. The hepato-protective
activity of curcumin for the treatment of NAFLD has been
evaluated in multiple clinical trials. Curcumin significantly
reduced total glycerides and waist circumference compared with
the placebo subgroup. It may become a promising agent in NAFLD
non-alcoholic fatty liver disease treatment.
Curcuma longa: A Natural Ally in Alzheimer’s Disease
Management
Curcumin and Neurodegenerative
Diseases | June 2024
Curcuma longa contains a
compound called curcumin that possesses potential
biopharmacological activity. Curcumin is considered safe by the
American Food and Drug Administration (FDA) and does not exhibit
any side effects when consumed in moderate amounts. Numerous
studies have been conducted on curcumin due to its therapeutic
potential in various diseases, particularly in neurodegenerative
disorders such as Alzheimer’s disease. Research indicates that
this substance holds remarkable potential as an effective and
safe treatment.
Patient-reported
outcomes of curcumin supplementation in rheumatoid arthritis and
psoriatic arthritis: a cross-sectional survey
Rheumatology International | June 2024
Pain scores decreased
significantly after starting curcumin therapy. Patients who were
taking curcumin for years reported better symptomatic control
when compared with patients taking it for months (p 0.01), weeks
(p 0.02), or days (p 0.02). There was a significant difference
in symptom improvement in patients taking 200–1000 mg compared
to patients taking less than 200 mg (p 0.01). Patients taking
curcumin once or twice a day reported significant symptom
improvement compared to patients taking it sporadically.
Symptomatic improvement was reported as pain (35.7%), swelling
(25%), stiffness (23.21%), and fatigue (16.07%). An interesting
correlation exists between the symptom relief and the frequency,
dosages (200–1000 mg), and duration (years) of curcumin
supplementation. Our study indicates that curcumin
supplementation positively influenced outcomes in 46.4% of
individuals with rheumatoid arthritis and psoriatic arthritis,
reducing pain, swelling, stiffness, and fatigue. This suggests
curcumin’s potential as an adjunct therapy for these conditions.
Curcumin Inhibits the Progression of Non-small Cell
Lung Cancer by Regulating DMRT3/SLC7A11 Axis
Molecular Biotechnology | June 2024
Emerging studies have
shown that curcumin might repress non-small cell lung cancer
progression by regulating ferroptosis. Curcumin blocked
non-small cell lung cancer cell proliferation and angiopoiesis,
and induced apoptosis and ferroptosis. DMRT3 or SLC7A11
upregulation partly abolished the suppressive role of curcumin
on non-small cell lung cancer development. In mechanism, DMRT3
was a transcription factor of SLC7A11 and increased the
transcription of SLC7A11 via binding to its promoter region.
Curcumin inhibited non-small cell lung cancer growth in vivo by
modulating DMRT3. Curcumin might constrain non-small cell lung
cancer cell malignant phenotypes partly through the
DMRT3/SLC7A11 axis, providing a promising therapeutic strategy
for non-small cell lung cancer.
Curcumin,
Piperine and Taurine Combination Enhances the Efficacy of
Transarterial Chemoembolization Therapy in patients with
Intermediate Stage Hepatocellular Carcinoma
Asian Pacific Journal of Cancer Prevention | June 2024
This
study aimed to assess the effectiveness of curcumin (C),
piperine (P) and taurine (T) combination as adjuvant agents on
serum levels of IFN-γ, immunophenotypic and molecular
characterization of mononuclear leukocytes (MNLs) in
hepatocellular carcinoma patients treated with Transarterial
chemoembolization (TACE). Patients and methods: Serum and MNLs
were collected from 20 TACE-treated hepatocellular carcinoma
patients before (baseline-control samples) and after treatment
with 5 g curcumin capsules , 10 mg piperine and 0.5 mg taurine
taken daily for three consecutive months. Immunophenotypic and
molecular characterization of MNLs were determined by flow
cytometry and quantitative real time PCR, respectively. In
addition, serum IFN-γ level was quantified by ELISA. Results:
After receiving treatment with curcumin piperine T
combination, there was a highly significant increase in IFN- γ
levels in the sera of patients when compared to basal line
control samples. Additionally, the group receiving combined
therapy demonstrated a downregulation in the expression levels
of PD-1, in MNLs as compared to controls. MNLs’
immunophenotyping revealed a significant decline in
CD4+CD25+cells (regulatory T lymphocytes). Furthermore,
clinicopathological characteristics revealed a highly
significant impact of curcumin piperine T combination on
aspartate aminotransferase (AST), lactate dehydrogenase (LDH)
and alpha feto protein (AFP) levels. This study introduces a
promising adjuvant curcumin piperine T combined treatment
as natural agents to enhance the management of hepatocellular
carcinoma patients who are candidates to TACE treatment.
The
effect of 8 weeks of HIIT training and Curcumin supplementation
on Adiponectin levels and insulin resistance in obese women with
type 2 diabetes
Journal of Sport and
Biomotor Sciences | June 2024
Exercise intervention three
sessions a week and daily curcumin consumption of 2100 mg in
three meals was implemented for 8 weeks. Results: The
interactive effect of curcumin and intense interval training
increases adiponectin (P=0.001), decreases insulin resistance
(P=0.001), decreases insulin (P=0.001), and significantly
decreases glucose (P=0.002). Conclusion: The current research
shows that performing intense intermittent exercises along with
curcumin as a non-invasive method can have a positive and
important effect on increasing adiponectin and reducing insulin
resistance in patients with type 2 diabetes.
Epigenetic Orchestration of Neurodegenerative
Disorders: A Possible Target for Curcumin as a Therapeutic
Neurochemical Research | June 2024
Studies have highlighted
the marked antioxidant and neuroprotective abilities of
polyphenols such as curcumin, by increased activity of
detoxification systems like superoxide dismutase (SOD), catalase
or glutathione peroxidase. The role of curcumin as an epigenetic
modulator in neurological disorders and neuroinflammation apart
from other chronic diseases have also been reported by a few
groups. This review summarizes the current knowledge of the role
of mitochondrial dysfunction, epigenetic modulations and
mitoepigenetics in age-associated neurological disorders such as
Alzheimer’s (AD), Parkinson’s (PD), Huntington’s disease (HD),
Amyotrophic Lateral Sclerosis (ALS), and Multiple Sclerosis
(MS), and describes the neuroprotective effects of curcumin in
the treatment and/or prevention of these neurodegenerative
diseases by regulation of the epigenetic machinery.
Effect of curcumin supplementation on symptoms of
anxiety: A systematic review and meta-analysis of randomized
controlled trials
Clinical Nutrition ESPEN
| June 2024
Curcumin is a polyphenolic natural compound that
has been used to treat various ailments such as symptoms of
anxiety. A total of eight RCTs involving 567 participants were
included in the analysis. A pooled analysis showed a significant
effect of curcumin on anxiety symptoms (SMD: -1.56; 95% CI:
-2.48, -0.64, p< 0.001; I2= 95.6%, p-heterogeneity< 0.001).
Present meta-analysis demonstrated that curcumin intake might
contribute to alleviation of anxiety disorder.
Turmeric: from spice to cure. A review of the
anti-cancer, radioprotective and anti-inflammatory effects of
turmeric sourced compounds
Frontiers in
Nutrition | June 2024
Extensive research, encompassing
preliminary, preclinical, and clinical studies, underscores the
pharmacological significance of curcumin, the yellow pigment in
turmeric. Its versatile properties include anti-inflammatory,
immunomodulatory, antioxidant, hypolipidaemic, antimicrobial,
anticarcinogenic, antitumor, radioprotective, neuroprotective,
hepato-protective, nephroprotective, cardio-protective, and
vasoprotective activities. Curcumin’s impact extends to various
biochemical pathways, influencing molecular targets such as
cytokines, transcription factors, kinases, growth factors, and
microRNAs. ecognizing the valuable insights from traditional
medicine in guiding natural product-based drug discovery,
researchers explore the medicinal applications of turmeric
across different traditional systems and investigate the modern
pharmacological activities of curcumin, bridging the knowledge
from ancient practices to current clinical trials. Through its
antioxidant properties and the down-regulation of inflammatory
targets, curcumin emerges as a promising agent in managing
inflammatory skin diseases.In summary, our review has uncovered
the multifaceted potential of curcumin both as an
immunomodulator, as a radioprotective, anticancer medication and
so much more. Curcumin boasts multiple benefits and presents
itself as an interesting subject for future research.
Therapeutic Role of Turmeric in the Management of
Periodontitis: A Comprehensive Review
Journal of Emerging Technologies and Innovative Research | June
2024
Curcumin stands out as a key bioactive compound,
recognized for its distinctive yellow color and a wide range of
beneficial biological effects. Curcumin has shown its prowess in
fighting inflammation by slowing down the growth of inflammatory
cells, curbing their spread, and inhibiting the formation of new
blood vessels. Curcumin is like a versatile player on the
chemical stage, boasting three highly reactive groups: one
diketone and two phenolic groups. These features allow curcumin
to engage in a range of chemical interactions that supercharge
its effectiveness. From reversible and irreversible reactions to
enzymatic processes and hydrogen transfers, curcumin can do it
all, enhancing its potency. Moreover, it's not just about its
own reactions—curcumin can also form sturdy bonds with both
metals and nonmetals, acting as a reliable agent for complexing
molecules. These unique traits make curcumin a valuable player
in various biological roles, demonstrating its adaptability and
impact. The extensive body of research surrounding
curcumin illuminates its remarkable anti-inflammatory
properties, offering a beacon of hope in the fight against
periodontal diseases. Through its ability to inhibit
inflammatory cell proliferation, curb their spread, and disrupt
blood vessel formation, curcumin presents a promising avenue for
reducing inflammation associated with periodontitis.
The Effect of Curcumin on Oxidative Stress and
Inflammatory Markers in Recreationally Active Women and Men
Virginia Polytechnic Institute and State University | June 2024
Curcumin, a bioactive compound found in turmeric, has been
linked to antioxidant and anti-inflammatory properties.
Curcumin, a compound found in turmeric, renowned for its
antioxidant and anti-inflammatory properties, presents a
potential avenue for managing oxidative stress and inflammation.
This study assessed whether a four-week regimen of turmeric
supplementation can attenuate markers of oxidative stress and
inflammation in physically active individuals, 18 to 45 years of
age. By investigating the potential antioxidant and
anti-inflammatory properties of curcumin, this research aimed to
contribute novel insights into strategies for mitigating
oxidative stress and inflammation, thereby promoting health,
well-being, and athletic performance.
Is Turmeric Beneficial To Diabetics? A Review By
Nutrition Professionals
MSN.com | June 2024
Curcumin, a compound present in turmeric, has anti-inflammatory,
antioxidant, anticancer and antimicrobial properties that help
improve blood glucose levels. Another benefit is that it has
cardioprotective, nephroprotective and hepatoprotective effects,
which are organs that are at risk in the presence of the
disease.Scientific evidence suggests that turmeric can help
people with diabetes. According to research, turmeric helps
control blood sugar levels, improve glucose sensitivity, and aid
in weight loss.
Research progress on curcumin improving chronic low-grade
inflammation and related diseases
Journal
of Chinese Materia Medica | June 2024
Chronic low-grade
inflammation(CLGI), a relatively new concept without a clear
definition, refers to a nonspecific, chronic, continuous, and
low-grade inflammation state, and it is closely associated with
various chronic diseases, including obesity, inflammatory bowel
disease, neurodegenerative diseases, and tumors. Improvement of
CLGI can slow down disease progression. Anti-inflammatory
treatment is an important strategy for prevention and treatment
of CLGI. However, there is currently no definitive drug
treatment method. Curcumin is a polyphenolic compound extracted
from the rhizome of zingiberaceae, with significant
anti-inflammatory activity. Research has shown that curcumin can
play an anti-inflammatory role by regulating NF-κB, JAK/STAT,
PI3K/Akt, MAPK, NLRP3 inflammasome, Nrf2/ARE, and other
inflammation-related pathways. This paper summarized the
anti-inflammatory mechanisms, pharmacological effect, and
clinical application of curcumin in improving CLGI and other
diseases, so as to provide a reference for in-depth research and
clinical application of curcumin in improving CLGI.
Curcumin Alone and Combined With PI3K Inhibitors
Elicits Positive Effects on Oropharyngeal Cancer Cell Lines
Anticancer Research | June 2024
Curcumin led to
dose-dependent responses with reduced viability and
proliferation; upon combining it with BYL719, additional
positive effects were found for most OPSCC lines grown as
monolayers, and these effects were validated in CU-OP-2 cells
grown as spheroids. Curcumin with MK-1775 or PD-0332991 also
elicited some positive effects on CU-OP-2 and CU-OP-17 cells.
Curcumin alone led to dose-dependent responses and when combined
with BYL719, positive effects were revealed, as they were when
it was combined with MK-1775 or PD-0332991, suggesting a
potential use of some of these combinations for HPV+ OPSCC.
Identification of Curcumin Targets in the Brain of
Epileptic Mice Using DARTS
ACS Omega | June
2024
Curcumin, a compound derived from turmeric, is
traditionally utilized in East Asian medicine for treating
various health conditions, including epilepsy. Despite its
involvement in numerous cellular signaling pathways, the
specific mechanisms and targets of curcumin in epilepsy
treatment have remained unclear. Our study focused on
identifying the primary targets and functional pathways of
curcumin in the brains of epileptic mice. Using drug affinity
responsive target stabilization (DARTS) and affinity
chromatography, we identified key targets in the mouse brain,
revealing 232 and 70 potential curcumin targets, respectively.
Bioinformatics analysis revealed a strong association of these
proteins with focal adhesions and cytoskeletal components.
Further experiments using DARTS, along with immunofluorescence
staining and cell migration assays, confirmed curcumin’s ability
to regulate the dynamics of focal adhesions and influence cell
migration. This study not only advances our understanding of
curcumin’s role in epilepsy treatment but also serves as a model
for identifying therapeutic targets in neurological disorders.
Curcumin, a plant polyphenol derived from the rhizomes of ginger
family plants such as Curcuma longa, has been confirmed to have
potential efficacy and safety in the treatment of epilepsy
through multiple clinical studies. Notably, curcumin therapy
does not interfere with the absorption, digestion, metabolism,
or excretion of other antiepileptic drugs (such as sodium
valproate, phenytoin, phenobarbital, and carbamazepine), and it
can enhance their therapeutic effects while reducing the dosage
and side effects.8 Currently, genomics-based drug repurposing
techniques have identified curcumin as one of the most promising
natural candidates for antiepileptic medication. Curcumin is a
compound derived from turmeric, a plant that has been
traditionally used to treat epilepsy. Our findings indicate that
curcumin affects the cell migration and cytoskeleton through
potential targets such as USP5, TNR, and CADPS.This system aims
to identify potential targets for curcumin, a promising
treatment for epilepsy. Our findings have revealed that USP5,
CADPS, and TNR are the potential target proteins for curcumin.
Curcumin in the treatment of inflammation and
oxidative stress responses in traumatic brain injury: a
systematic review and meta-analysis
Frontiers in Neurology | June 2024
In our study, curcumin
exhibits potent anti-inflammatory, antioxidant, and
anti-apoptotic effects, suggesting its potential for treating
TBI in humans. Curcumin, a neuroprotective agent, is relatively
underexplored in TBI research, making our topic selection novel.
Curcumin, an active compound extracted from the root of Curcuma
longa, has demonstrated neuroprotective properties both in vitro
and in vivo. Notably, it has shown potential in reducing
oxidative stress and inflammation and enhancing redox balance.
The analysis revealed that curcumin significantly reduced
inflammatory cytokines across various concentrations, time
points, and administration routes. Additionally, curcumin
markedly enhanced the activity of oxidative stress markers while
reducing MDA and oxyprotein levels. Furthermore, curcumin
improved cerebral edema and upregulated neuroprotective factors
like synapsin I, BDNF, and CREB, without reducing mNSS. About
autophagy and apoptosis, curcumin increased the activity of
Beclin-1and Bcl-2, while decreasing caspase-3, the apoptosis
index, and P62. Curcumin supplementation positively
affects traumatic brain injury (TBI) by alleviating oxidative
stress and inflammatory responses and promoting neuroprotection.
It holds potential as a therapeutic agent for human TBI.
The Role of Curcumin in Oral Health and Diseases: A Systematic
Review
Antioxidants | May 2024
Curcumin
is a prominent natural compound that has been extensively
studied in the recent literature due to its anti-inflammatory,
antioxidant, antibacterial, and anticancer activities. Since
ancient times, curcumin has been recognized for its multiple
therapeutic properties, which have been confirmed by recent
clinical studies. Recent scientific research on curcumin has
demonstrated that it possesses the following properties:
anti-inflammatory, antioxidant, antifungal, antidepressant,
healing (therapeutic in Crohn’s disease, ulcerative colitis, and
peptic and gastric ulcers), and as an adjuvant in acute coronary
syndrome therapies, chemotherapies, and as a hypoglycemic.
Curcumin also stands out for its ability to positively influence
intestinal microbiota, thereby contributing to maintaining an
optimal microbial balance. This effect can have significant
implications in the prevention and treatment of gastrointestinal
disorders, thereby enhancing overall health. Additionally,
recent studies have highlighted curcumin has potential in
supporting brain health and cognitive function, opening
intriguing avenues in the treatment of neurodegenerative
disorders, such as Alzheimer’s and Parkinson’s diseases.
Curcumin also protects against risk factors such as smoking,
alcohol, and stress, The ascertained pleiotropic nature of
curcumin creates interference in complex biological processes
and various inflammatory factors that regulate
oxidation–reduction processes, such as reactive oxygen species
(ROS), cytokines, cyclooxygenase-2 (COX-2), interleukins (ILs),
nuclear factor kappa B (NF-κB), C-reactive proteins,
transforming growth factor-β (TGF-β), and other enzymes involved
in inflammation. Curcumin inhibits vascular endothelial growth
factor (VEGF) and various kinases, demonstrating a controlling
action in angiogenesis and the growth of cancerous lesions. Due
to its medicinal and pharmacological properties, curcumin has
also found application in the treatment of multiple diseases of
the oral cavity (caries, periodontitis, gingivitis, aphthous
stomatitis, oral candidiasis, mucositis, oral submucosal
fibrosis, lichen planus, oral leukoplakia, carcinogenic lesions,
etc.), which affect approximately 3.5 billion people worldwide.
The regulating effect of curcumin on NF-κB pathway
in neurodegenerative diseases: a review of the underlying
mechanisms
Inflammopharmacology | May 2024
Curcumin has been noted to be a popular anti-oxidant and
anti-inflammatory substance and is the foremost natural compound
produced by turmeric. According to various studies, when playing
an anti-inflammatory role, it interacts with several modulating
proteins of long-standing disease signaling pathways and has an
unprovocative consequence on pro-inflammatory cytokines. This
review article determined to figure out curcumin’s role in
limiting the promotion of neurodegenerative disease via
influencing the NF-κB signaling route. Preclinical studies were
gathered from plenty of scientific platforms including PubMed,
Scopus, Cochrane, and Google Scholar to evaluate this
hypothesis. Extracted findings from the literature review
explained the repressing impact of Curcumin on the NF-κB
signaling pathway and, occasionally down-regulating the cytokine
expression.
Antiviral, anti-inflammatory and antioxidant effects
of curcumin and curcuminoids in SH-SY5Y cells infected by
SARS-CoV-2
Nature | May 2024
Curcumin, a
natural spice, has garnered significant attention for its
potential in treating conditions characterized by immune system
perturbations and inflammatory responses, including COVID-19.
CUR and other curcuminoids are the primary bioactive components
of turmeric (Curcuma longa), a substance that has been
employed in the traditional medicine practices of diverse
cultures for centuries. This enduring use is attributed to the
anti-inflammatory, antioxidant, antibacterial, antiviral and
neuroprotective properties exhibited by curcuminoids.
Furthermore, curcuminoids can play a role in inhibiting certain
enzymes associated with the inflammatory process, such as
mitogen-activated protein kinases (MAPKs), c-Jun N-terminal
kinases (JNK), and nuclear factor kappa B (NF-kB). The
antioxidant properties of curcuminoids have been shown to
inhibit carcinogenic reactive oxygen species (ROS), including
superoxide anions, hydroxyl radicals, nitrites, and peroxides.
Curcumin can stimulate both the activation of antioxidant
enzymes and the decrease in expression of pro-inflammatory
cytokines. A recent systematic review identified six studies
demonstrating that curcumin supplementation led to a significant
decrease in common COVID-19 symptoms, reduced hospitalization
duration, and decreased mortality rates. The authors concluded
that curcumin treatment mitigates the manifestation of cytokine
storms by reducing pro-inflammatory factors and activating
anti-inflammatory pathways. They further suggested that curcumin
treatment may alleviate COVID-19 symptoms by restoring the
balance between pro-inflammatory and anti-inflammatory
responses.
Curcumin Promotes the Proliferation, Migration, and
Angiogenesis of HUVECs to Improve Atherosclerosis through the
Wnt/β-Catenin Pathway
Journal of Biological
Regulators and Homeostatic Agents | May 2024
Curcumin
treatment significantly alleviated ox-LDL-induced HUVECs injury,
as evidenced by elevating the proliferative ability, cell
migration, and angiogenesis. Moreover, the Wnt/β-catenin pathway
was substantially boosted following ox-LDL treatment, which was
suppressed by curcumin. The inhibitory effects of curcumin
treatment on the Wnt/β-catenin signaling pathway were reduced by
SKL2001. Furthermore, the promoting effects of curcumin on
ox-LDL-induced cell damage were hampered following SKL2001
treatment in HUVECs (p < 0.05). Conclusion: Curcumin elevated
cell proliferation, migration, and angiogenesis of HUVECs to
inhibit the development of atherosclerosis through inactivating
the Wnt/β-catenin pathway.
The
Impact of Curcumin, Resveratrol, and Cinnamon on Modulating
Oxidative Stress and Antioxidant Activity in Type 2 Diabetes:
Moving beyond an Anti-Hyperglycaemic Evaluation
Antioxidants | May 2024
Curcumin is an active
chemical compound present in the rhizome of the plant Curcuma
longa, also known as turmeric. It exhibits antioxidant,
anti-inflammatory, antimicrobial, anticancer, anti-rheumatic,
immunomodulatory, anti-hyperglycaemic, and cardio-renal-hepato-protective
properties. In one animal study, curcumin and its analogues were
shown to have a similar mechanism of action to
thiazolidinedione, an antidiabetic drug, through the activation
of the peroxisome proliferator-activated receptor gamma
(PPAR-γ), suggesting that curcumin may be effective in
regulating glycaemia and lipid levels. Curcumin appears to have
beneficial effects in reducing fasting glucose, glycated
haemoglobin (HbA1c), homeostatic model assessment of insulin
resistance (HOMA-IR), and TNF-α. It also positively influences
lipid metabolism by lowering low-density lipoprotein (LDL)
cholesterol and triglyceride levels while increasing
high-density lipoprotein (HDL) cholesterol. Curcumin
demonstrates beneficial effects not only on the glycaemic
control but also on anthropometric parameters. Hodaei et al.
recruited 53 individuals with excess body weight and T2DM. After
10 weeks of supplementation, the study group showed a
significant decrease in body weight, body mass index (BMI),
waist circumference, and blood glucose levels (p < 0.05)
compared to the control group. Jiménez-Osorio et al investigated
the impact of curcumin supplementation on markers of oxidative
stress, antioxidant enzyme activity, and nuclear factor
erythroid 2-related factor 2 (Nrf2) activation. In individuals
with non-diabetic proteinuria and CKD, curcumin attenuated lipid
peroxidation, whereas curcumin increased antioxidant capacity in
individuals with T2DM and CKD with proteinuria. These results
suggest a potential reduction in oxidative stress in patients
with diabetes and CKD. Patients with T2DM and CKD (n = 14)
received 500 mg/day of curcumin for 15 days (30 days in patients
with overt proteinuria). Curcumin supplementation significantly
reduced urinary microalbumin excretion and lowered serum MDA
levels by enhancing the specifically Nrf2-regulated protein,
NAD(P)H quinone oxidoreductase 1 (NQO-1), along with other
antioxidant enzymes in the lymphocytes. Additionally, patients
showed reduced serum lipopolysaccharide (LPS) content and
increased inhibitor of nuclear factor kappa-B kinase (IκB
kinase) proteins inhibiting inflammatory signalling in
lymphocytes. Interestingly, curcumin stimulated the activity of
several gut bacteria crucial for maintaining the integrity and
functioning of the gut barrier. In summary, short-term curcumin
intervention inhibits the progression of DKD by activating the
Nrf2 antioxidant system and exerting anti-inflammatory effects
in patients with T2DM. Aerobic training + curcumin
supplementation group showed the best results with significantly
reduced MDA and hs-CRP levels and increased glutathione and TAC
compared to the aerobic-training and
curcumin-supplementation-alone, respectively. These results
demonstrate the positive effects of curcumin supplementation and
physical activity on the metabolic state, oxidative stress
markers, and hs-CRP. Curcumin supplementation appears to have
beneficial effects in individuals with T2DM and coronary heart
disease. Shafabakhsh et al. recruited 60 patients with T2DM and
CHD aged 45–85 and were randomly assigned to two groups—the
experimental group receiving 1000 mg/day of curcumin and the
control group receiving a placebo for 12 weeks. After the
intervention, the experimental group showed a significant
reduction in MDA levels and a significant increase in TAC and
GSH levels compared to those of the placebo group. Additionally,
curcumin consumption increased the level of peroxisome
proliferator-activated receptor gamma (PPAR-γ). Na et al.
investigated whether curcumin supplementation reduced A-FABP
levels. The curcumin-receiving group had significantly reduced
serum A-FABP levels, CRP, TNF-α, and IL-6compared to the placebo
one. Additionally, curcumin supplementation also significantly
increased the activity of superoxide dismutase (SOD) in serum.
The researchers concluded that curcumin may have antidiabetic
effects by reducing A-FABP levels in serum. Curcumin also
exhibits lipid-metabolism-lowering effects. Forty-four
individuals with T2DM were randomly assigned to a group
supplementing with curcumin at a dose of 1500 mg/day or to a
control group receiving a placebo for 10 weeks. In the
curcumin-supplementing group, the average hs-CRP concentration
significantly decreased, and the average adiponectin
concentration significantly increased compared to in the placebo
group. These results suggest that curcumin intake may reduce
diabetic complications by decreasing TG levels and inflammatory
markers.
Unraveling the protective effects of curcumin
against drugs of abuse
Cell | May 2024
Curcumin, a natural compound derived from the turmeric plant (Curcuma
longa), has garnered significant attention for its diverse
neuroprotective properties. Curcumin has been widely recognized
for its remarkable anti-inflammatory, antioxidant, and
anti-apoptotic effects, which have shown great potential in the
treatment of various disorders, encompassing psychiatric and
neurodegenerative diseases. Overall, curcumin demonstrates
promising effects against the neurotoxicity induced by abused
drugs through a wide range of mechanisms; modulation of
inflammatory cytokines, maintenance of ion homeostasis,
epigenetic regulation, enhancement of antioxidant capacity, as
well as the activation of the cAMP response element-binding
protein (CREB) and brain-derived neurotrophic factor (BDNF)
signaling pathways. Curcumin has demonstrated its viability as a
potential therapeutic option for cancer treatment by selectively
targeting diverse cell signaling pathways encompassing growth
factors, cytokines, transcription factors, and genes that
regulate cellular proliferation and apoptosis. Curcumin has been
shown to be effective in treating outcomes linked to obesity
through promoting the expression of antioxidants and directly
slowing preadipocyte development. Studies have demonstrated the
possible benefits of curcumin in chronic obstructive pulmonary
disease (COPD), through attenuation of airway inflammation and
remodeling. Moreover, the relaxant effects of curcumin on
tracheal smooth muscle indicate its bronchodilatory properties,
suggesting its potential advantage in the treatment of diverse
respiratory and allergic disorders. This evidence supports its
ability to promote crucial aspects of wound healing, such as
collagen deposition, granulation tissue formation, tissue
remodeling, and wound contraction. Another potential mechanism
of action of curcumin on depressive symptoms is linked to the
inhibition of transcription signaling pathways of some nuclear
factors, including nuclear factor kappa B, which is necessary
for the production of pro-inflammatory cytokines and is
consequently involved in the pathogenesis of neuroinflammation.
Furthermore, curcumin has been shown to boost levels of
brain-derived neurotrophic factor (BDNF), a neurotrophin linked
to the etiology of depression. In addition to its therapeutic
effects on depressive disorders, curcumin has exhibited
potential as a therapeutic intervention for delusional
disorders. These findings suggest that curcumin emerges as a
promising therapeutic agent in combatting the detrimental
effects induced by drugs of abuse. Curcumin has demonstrated the
ability to attenuate neuronal damage, oxidative stress,
inflammation, and apoptosis induced by these addictive
substances. The findings presented in this review highlight the
potential of curcumin as a multifaceted neuroprotective agent in
the context of drug addiction. By targeting key pathological
pathways associated with drug-induced neurotoxicity, curcumin
holds great promise for the prevention and treatment of
addiction-related cognitive impairments and neurodegenerative
disorders. Furthermore, the ability of curcumin to modulate
neuroplasticity and synaptic plasticity provides a rationale for
its use in promoting neuronal recovery and functional
restoration following chronic drug exposure.
Curcumin in the treatment of inflammation and
oxidative stress responses in traumatic brain injury: a
systematic review and meta-analysis
Frontiers in Neurology | May 2024
Curcumin, an active
compound extracted from the root of Curcuma longa, has
demonstrated neuroprotective properties both in vitro
and in vivo. Notably, it has shown potential in
reducing oxidative stress and inflammation and enhancing redox
balance. Curcumin supplementation positively affects traumatic
brain injury by alleviating oxidative stress and inflammatory
responses and promoting neuroprotection. It holds potential as a
therapeutic agent for human traumatic brain injury.
Curcumin significantly mitigates the effects of inflammatory
cytokines such as IL-1β, IL-6, and TNF-α. Moreover, curcumin
enhances the efficacy of oxidative stress factors, including
SOD, Sir2, GPx, and Nrf2. Regarding neurological function,
curcumin reduces brain edema, increases neuron survival rates
and augmentation. Furthermore, curcumin enhances the effects of
Beclin-1 and Bcl-2 in autophagy and apoptosis. Curcumin,
administered in various concentrations, durations, and routes,
significantly reduces pro-inflammatory cytokines in the
experimental group, improves nerve function following TBI, and
mitigates the inflammatory response. Bassani et al. demonstrated
that curcumin effectively reduces neuroinflammation in models of
neurodegenerative and neuroinflammatory diseases. Additional
studies conducted in animal experimental models have shown that
curcumin inhibits the TLR4/NF-κB signaling pathway, thereby
down-regulating inflammatory cytokines. The results of this
meta-analysis confirm that curcumin effectively reduces
oxidative stress levels following TBI. Curcumin, a widely used
antioxidant with neuroprotective properties, has been shown to
inhibit lipid peroxide formation in the presence of lipid
peroxidation-inducing drugs. Moreover, curcumin reduces levels
of MDA, 4-HNE, and protein carbonyls, restores mitochondrial
oxidation function, stabilizes cell membrane homeostasis, and
thereby mitigates the oxidative stress response following TBI.
Gao et al. demonstrated that curcumin treatment significantly
reduces MDA levels and induces GPx activation, thereby
ameliorating TBI-induced oxidative stress in rat models. Studies
provide evidence that curcumin is a potent activator of Nrf2
both in vivo and in vitro, enhancing Nrf2
activation in the brain.
Curcumin exerts anti-inflammatory, antioxidant and
anti-ferroptotic effects through the Nrf2/HO-1 pathway to
protect cardiomyocytes against sepsis
Signa
Vitae | May 2024
The results show that curcumin significantly
improved cell viability in lipopolysaccharide (LPS)-induced H9c2
cells (p < 0.01). Curcumin also significantly reduced
inflammatory factor levels in LPS-induced cardiomyocytes (p <
0.001). Curcumin down-regulated ROS and MDA levels (p < 0.001),
and up-regulated SOD and GSH levels (p < 0.001). A decrease in
both Fe2+ content and protein expression of ACSL4 (p < 0.001),
and increased protein expression of glutathione peroxidase 4
(GPX4) (p < 0.001) were observed with curcumin. By knocking down
Nrf2 curcumin’s therapeutic effect against LPS was eliminated.
So curcumin can inhibit LPS-induced oxidative stress,
inflammation and ferroptosis in cardiomyocytes by regulating
Nrf-2/HO-1 signaling.
The Role of Curcumin in Cancer: A Focus on the PI3K/Akt Pathway
Cancers | May 2024
Curcumin is a polyphenol isolated from the
rhizomes of Curcuma longa and has been widely studied for its
anti-inflammatory, anti-oxidant, and anti-cancer effects.
Curcumin acts on the regulation of different aspects of cancer
development, including initiation, metastasis, angiogenesis, and
progression. The phosphatidylinositol-3-kinase (PI3K)/protein
kinase B (AKT) pathway is a key target in cancer therapy, since
it is implicated in initiation, proliferation, and cancer cell
survival. Curcumin has been found to inhibit the PI3K/Akt
pathway in tumor cells, primarily via the regulation of
different key mediators, including growth factors, protein
kinases, and cytokines. This review presents the therapeutic
potential of curcumin in different malignancies, such as
glioblastoma, prostate and breast cancer, and head and neck
cancers, through the targeting of the PI3K/Akt signaling
pathway. This review focuses on the multifaceted role of
curcumin in improving different malignancies, primarily through
the regulation of the PI3K/Akt signaling pathway. From a
biological point of view, this structural property of curcumin
has been widely studied in diseases such as arthritis and
Alzheimer’s disease. Moreover, the presence of the diketone
group, along with the two phenolic groups, allows curcumin to
take part in different reactions in which electron transfer and
hydrogen abstraction are involved. This is particularly useful
in the case of reactions with free radicals. Curcumin’s reaction
with reactive oxygen species (ROSs) results in the formation of
curcumin–phenoxyl radicals that are more stable and less
reactive than the initial free forms. Interestingly, the
stabilization of superoxide radicals by curcumin has been
considered equally efficient to the effects of key antioxidants,
such as superoxide dismutase.
Curcumin attenuates brain aging by reducing
apoptosis and oxidative stress
Metabolic
Brain Disease | May 2024
Curcumin, exerts its neuroprotective
and anti-aging effects in the aged brain. Curcumin is a natural
antioxidant with potent anti-aging and neuroprotective
properties. Our results indicated that treatment with curcumin
in aged rats attenuates brain lipid peroxidation, which was
accompanied by a significant increase in the BDNF, VEGF,
superoxide dismutase (SOD) activity, and anti-apoptotic protein
BCl-2. The study indicates that curcumin could alleviate brain
aging which may be due to attenuating oxidative stress,
inhibiting apoptosis, and up-regulating SOD activity, which in
turn enhances VEGF and BDNF. Therefore, curcumin has potential
therapeutic value in the treatment of neurological apoptosis,
neurogenesis, and angiogenesis changes caused by brain aging.
Effect of Curcumin plus Piperine on Redox Imbalance
and Inflammation in Inflammatory Bowel Disease Patients: A
Randomized, Double-Blind, Placebo-Controlled Clinical Trial
Preprints | May 2024
Curcumin, a hydrophobic polyphenol,
exhibits potent antioxidant, anti-inflammatory, antitumor,
antimicrobial, anti-glycating, anti-coagulant and healing
properties, as evidenced by both experimental studies and
clinical trials clinical trials in IBD and other clinical
situations such as Type 2 diabetes and some cancers. Our
findings allow to affirm that the standard commercially
available powder, rich in curcumin and combined with piperine,
exhibits significant antioxidant action by substantially
enhancing the endogenous defense mechanism, as evidenced by a
noteworthy increase in serum SOD superoxide dismutas levels
among supplemented patients over the three-month intervention
period. These unprecedented results for this polyphenol
underscore its beneficial effects on patients with IBD. Results
are corroborated by a randomized clinical trial in patients with
metabolic syndrome, where the combination of curcumin plus
piperine, supplemented at the same dosage used daily in this
study (1000 mg + 10 mg), for 8 weeks, significantly increased
SOD superoxide dismutas activity. Another study conducted by
Panahi et al. also demonstrated the effectiveness of curcumin in
increasing SOD activity in patients with osteoarthritis after 6
weeks thus demonstrating the effectiveness of curcumin on this
marker. of redox imbalance. The observed increase in SOD levels
in this study among patients receiving curcumin plus piperine,
with no alterations in H2O2 and MDA levels, suggests a
beneficial effect of this supplementation.
Curcumin prevents high glucose-induced stimulatory
effects of renal cell secretome on fibroblast activation via
mitigating intracellular free radicals and TGF-β secretion
Biomedicine & Pharmacotherapy | May 2024
All the effects of
high glucose were successfully mitigated by curcumin. Curcumin
prevents the high glucose-induced stimulatory effects of renal
cell secretome on fibroblast activation, at least in part, via
mitigating intracellular ROS and TGF-β secretion. In kidney
stone mice and H2O2-exposed macrophages, curcumin has been
demonstrated to hamper oxidative stress by elevating glutathione
production and antioxidant enzyme activity. Additionally,
curcumin has been reported with an anti-fibrotic property to
attenuate renal fibrosis induced by kidney stone disease and
ischemia/reperfusion injury in mice accompanied by the reduction
of α-smooth muscle actin (α-SMA), collagen I, and fibronectin
expression. Curcumin has multiple health benefits due to its
anti-diabetic, anti-oxidative and anti-inflammatory effects.
Particularly in chronic kidney disease (CKD), recent randomized
controlled trials have reported that curcumin can reduce
oxidative stress as recognized by a decrease in malondialdehyde
(an oxidative stress marker) and an increase in catalase (an
antioxidant) in the plasma of CKD patients undergoing
hemodialysis and peritoneal dialysis. Furthermore, curcumin
supplementation for 12 weeks can suppress inflammatory processes
in hemodialysis patients as shown by reduced expression of NF-κB
in peripheral blood mononuclear cells and decreased plasma
levels of pro-inflammatory cytokines (IL-6 and TNF-α).
A role for curcumin in preventing liver fibrosis in
animal: A systematic review and meta-analysis
Frontiers in Pharmacology | May 2024
Curcumin therapy
improved fibrosis degree, oxidative stress level, inflammation
level, and liver synthesis function in animal models of liver
fibrosis. Curcumin intervention not only mitigates liver
fibrosis but also enhances liver function, while concurrently
modulating inflammatory responses and antioxidant capacity in
animal models. This result provided a strong basis for further
large-scale animal studies as well as clinical trials in humans
in the future. Numerous studies demonstrated that curcumin
effectively targets the inhibition of HSC activation (Shu et
al., 2023). Curcumin could inhibit activity and promote
apoptosis in LX-2 cells by suppressing autophagy through
activation of the PI3K/Akt/mTOR signaling pathway (Shu et al.,
2023). Lian et al. discovered that curcumin inhibits glycolysis
and regulates metabolism in HSCs by modulating hedgehog
signaling (Lian et al., 2015). Qin et al., in their intervention
with curcumin on HSC-T6 cells, found that curcumin could protect
against activation and migration of hepatic stellate cells by
inhibiting the CXCL12/CXCR4 biological axis in liver fibrosis
(Qin et al., 2018). Research indicates that curcumin reduces
serum cholesterol levels by increasing hepatic LDL receptor
expression, inhibiting LDL oxidation, and enhancing bile acid
secretion and faecal cholesterol excretion (Zhang et al., 2019).
Curcumin also suppresses genes involved in cholesterol
biosynthesis, proecting against liver injury and fibrogenesis in
animal models (Fu et al., 2008; Peschel et al., 2007). Curcumin,
a hydrophobic polyphenol extracted from the rhizome of
Curcuma longa, is widely utilized in the treatment of
cardiovascular diseases, liver diseases, and tumours (Xu et al.,
2020; Jabczyk et al., 2021; Pourbagher-Shahri et al., 2021).
Clinical and basic studies have confirmed its remarkable
pharmacological efficacy in treating liver diseases like NAFLD,
liver fibrosis, and liver cancer (Nelson et al., 2017; Farzaei
et al., 2018); (E. S. Lee et al., 2020) Our meta-analysis
showcased curcumin’s effectiveness in preclinical liver fibrosis
studies. The potential protective mechanisms observed in animals
encompass liver protection, collagen production inhibition,
oxidative stress reduction, and inflammatory response
regulation.
Curcumin alleviates myocardial inflammation, apoptosis, and
oxidative stress induced by acute pulmonary embolism
Journal of Physiology and Pharmacology | May 2024
Curcumin is
a substance extracted from the roots of the turmeric plant and
is a lipophilic polyphenol that acts as an antibiotic,
anti-inflammatory, and anti-aging agent. Curcumin has
significant tumor suppressor potential in a variety of cancers
by inhibiting cancer cell proliferation, metastasis, cell cycle
entry, or anti-apoptosis. Studies have determined that curcumin
is beneficial in regulating oxidative stress, inflammation, and
apoptosis in cardiovascular diseases. It has been described that
curcumin inhibits lung injury and inflammation in APE by
microRNA- 21/phosphatase and tensin homologue (PTEN) axis and
nuclear factor kappaB (NF-kB) pathway. In addition, curcumin
ameliorates oxidative stress and inhibits apoptosis in diabetic
cardiomyopathy. Moreover, it can reduce the production of
pro-inflammatory cytokines and alleviate myocardial injury. It
has been validated that curcumin has a protective effect on
cardiomyocytes by mediating oxidative stress and apoptotic
pathways. Curcumin acts against coronary microembolization-induced
myocardial damage by which the mechanism is related to reducing
myocardial apoptosis and inhibiting inflammation. The protective
effects of curcumin on cardiovascular diseases have been
extensively elucidated. Curcumin restores biochemical indices,
maintains antioxidant capacity, and reduces pro-inflammatory
cytokine in diabetes-induced myocardial infarction. Consistent
with these study findings, our research discovered that curcumin
pretreatment recovered pathological changes in myocardial tissue
and reduced apoptosis, inflammatory responses, and oxidative
stress by altering relevant indicators. Protective efficacy
against cardiovascular diseases by curcumin, a common natural
polyphenolic compound, which has antithrombotic properties and
reduces platelet accumulation in the circulation by inhibiting
thromboxane synthesis has been demonstrated. Curcumin improved
APE-induced myocardial injury, reduced myocardial tissue edema,
and thrombus volume. It attenuated APE-induced myocardial
inflammation and apoptosis, as well as reduced lung injury and
pulmonary artery pressure. Curcumin promoted microRNA-145-5p
expression in APE rat myocardium. In conclusion, curcumin
alleviates myocardial inflammation, apoptosis, and oxidative
stress induced by APE by regulating microRNA-145-5p/IRS1 axis.
Investigation
of Antibacterial Activity of Curcumin and Synergistic Effect
with Gentamicin Sulfate
Namık Kemal
University | May 2024
Curcumin is a food spice that is a
natural component of Curcuma longa rhizomes. It
has been widely used as a medicine in the treatment of various
diseases in Asian and Middle Eastern countries for years.
Curcumin, also known as turmeric, has been shown to have
antibacterial, antifungal, antiviral, antioxidant,
anti-inflammatory and anticancer activities. In the society, it
is known to be used for therapeutic purposes against various
malignant diseases, diabetes, arthritis, gastritis, urinary
tract infections, skin diseases and other chronic diseases.
Studies have shown that combinations of curcumin with different
agents, including various antibiotics, have synergistic effects
against bacteria. Curcumin is a natural agent whose therapeutic
effects have been investigated due to its various biological and
medicinal properties. In this study investigating the
antibacterial activity of curcumin on some standard strains, it
was found that curcumin showed antibacterial activity at a
concentration of 62.5 µg/mL against all tested bacteria except
E. faecalis (7.81 µg/mL). The results of this study and similar
studies also emphasize the antibacterial activity of curcumin.
In a study by Ungphaiboon et al. , in which they investigated
the antibacterial activity of curcumin extracts obtained from
Curcuma longa L. rhizomes on various microorganisms,
they found the MIC values of curcumin as 16 and 128 µg/mL for
Bacillus subtilis NCTC 10073 and S. aureus ATCC 25923,
respectively. This study shows that curcumin alone or in
combination with gentamicin sulfate has antimicrobial activity.
Curcumin inhibits colorectal cancer development by
blocking the YAP/TAZ signaling axis
Biocell
| May 2024
Curcumin is a plant polyphenol with antitumor
properties and inhibits the development of colorectal cancer. In
our study, we proved that curcumin significantly inhibited the
colorectal cancer cell viability, cell migration, and cell
invasion abilities. In addition, curcumin inhibited YAP and
Transcriptional coactivator with TAZ or the YAP/TAZ signaling
axis in colorectal cancer cells. Further, in the nude mice
model, curcumin treatment significantly decreased the size and
weight of xenotransplant tumors. Therefore, curcumin
significantly inhibited colorectal cancer development and
invasion by regulating the YAP/TAZ signaling axis. Curcumin has
documented various properties on tumor cells, including
anti-proliferative, anti-inflammatory, and antioxidant
activities. Studies have shown that curcumin could effectively
inhibit the invasion and proliferation of human cancers, such as
Wilms tumor (WT), esophageal cancer, and colorectal cancer .
Further, curcumin inhibits the occurrence and development of
colorectal cancer mainly through anti-inflammatory mechanisms
and anti-tumor-related pathways. Previous studies have shown
that curcumin has an inhibitory effect on colorectal cancer. Our
experimental results that curcumin inhibited the cell viability,
migration, and invasion of colorectal cancer in a dose-dependent
manner are also consistent with such reports. In conclusion,
this study demonstrates that curcumin inhibits the YAP/TAZ
signaling pathway by promoting YAP phosphorylation and
ultimately inhibits colorectal cancer tumorigenesis. Our study
also further clarified the mechanism of curcumin in the
treatment of colorectal cancer, laying an experimental and
theoretical basis for further elucidation of the mechanism of
curcumin in colorectal cancer treatment. This finding has
promising significance for promoting the application of curcumin
for treating colorectal cancer.
Therapeutic Potentials Of Curcumin Against
Neurodegenerative Diseases: A Review
International Journal Of Research and Analytical Reviews | May
2024
Curcumin, a polyphenolic compound found in turmeric, has
gained attention for its potential neuroprotective properties.
It has long been utilized as a medical herb and culinary spice
in Asian countries for a range of illnesses. This review centers
on the latest developments and the underlying processes of
curcumin's numerous natural benefits against neurodegenerative
diseases, including Parkinson's disease. The current research
indicates that the diagnosis of major depressive disorder is
related to both the neuroprotective benefits of antidepressants
and cellular shrinkage and the death of neurons. The
neuroprotection of curcumin against Parkinson's disease and
Alzheimer's disease is primarily attributed to its
anti-inflammatory and antioxidant properties. In addition,
according to reports curcumin improves striatal TH survival
filaments additionally, pars compacta (SNPC) neurons, reduces
aberrant turning geste, and exerts neuroprotective effects, at
least partially, through a 7-nAChR-mediated medium. In multiple
sclerosis (MS), an important part of the immune system is
inflammation that leads to the damage seen in the CNS. Curcumin
inhibits T cell, and IL-12 signaling and may be used to treat MS
and other inflammatory conditions. Curcumin’s anti-inflammatory,
anticancer, and antibacterial qualities have been the subject of
numerous both in vitro and in vivo investigations, both of its
own and in conjunction with conventional therapies. Studies on
epidemiology have shown that cultures with high curcumin
consumption have lower rates of disorders affecting inflammatory
and memory retention, such as Alzheimer's illness. Curcumin has
been utilized and additionally proposed is a possible preventive
measure against Parkinson's disorder since studies have
indicated that curcumin-consuming Indian communities do not
exhibit age-related alterations in their neural neurons that
produce dopamine. Furthermore, as will be covered in more
information below, a multitude of both vivo and in vitro
investigations offer strong proof that curcumin protects against
Parkinson's disease-like symptoms. In conclusion, the
neuroprotective effect of curcumin on neurodegenerative diseases
holds significant promise based on the comprehensive body of
research conducted thus far. Through its multifaceted mechanisms
of action including antioxidant, anti- inflammatory, and
anti-amyloid properties, curcumin demonstrates the potential to
mitigate neurodegenerative processes associated with conditions
such as Depression, Alzheimer's disease, Parkinson's disease,
and multiple sclerosis. The studies reviewed consistently
indicate that curcumin possesses the ability to modulate key
cellular pathways involved in neuroprotection, thereby
attenuating neuronal damage and improving cognitive function in
various experimental models. The existing evidence underscores
curcumin as a promising natural compound worthy of continued
exploration for the development of novel therapeutic strategies
against neurodegenerative diseases.
Patient-reported outcomes of curcumin
supplementation in rheumatoid arthritis and psoriatic arthritis:
a cross-sectional survey
Rheumatology
International | April 2024
Pain scores decreased
significantly after starting curcumin therapy. Patients who were
taking curcumin for years reported better symptomatic control
when compared with patients taking it for months, weeks, or
days. There was a significant difference in symptom improvement
in patients taking 200–1000 mg compared to patients taking less
than 200 mg. Patients taking curcumin once or twice a day
reported significant symptom improvement compared to patients
taking it sporadically. An interesting correlation exists
between the symptom relief and the frequency, dosages
(200–1000 mg), and duration (years) of curcumin supplementation.
Our study indicates that curcumin supplementation positively
influenced outcomes in 46.4% of individuals with rheumatoid
arthritis and psoriatic arthritis, reducing pain, swelling,
stiffness, and fatigue. Curcumin is suggested to possess potent
anti-inflammatory properties. This suggests curcumin’s potential
as an adjunct therapy for these conditions.
Curcumin
Inhibits α-Synuclein Aggregation by Acting on Liquid–Liquid
Phase Transition
Food | April 2024
Parkinson’s disease, the second most common neurodegenerative
disorder, is linked to α-synuclein (α-Syn) aggregation. Curcumin
can inhibit amyloid formation by inhibiting the occurrence of
LLPS and the subsequent formation of oligomers of α-Syn in the
early stages of aggregation. These results may help to clarify
the mechanism by which curcumin inhibits the formation of α-Syn
aggregates during the development of Parkinson’s disease.
Curcumin, the main polyphenolic substance present in the
rhizomes of Curcuma longa L., has been shown to
interact with amyloid-β peptide and α-Syn, thus inhibiting their
aggregation, deposition, and neurotoxicity. Importantly,
curcumin has also been recognized for its pharmacological
benefits in a multitude of pathological contexts, including
diabetes, cancer, and even neurodegenerative diseases. We found
that curcumin could inhibit the formation of α-Syn oligomers by
affecting the initial phase transition of α-Syn in the
condensation pathway through direct interaction with droplets.
Neuroprotective and anti-inflammatory effects of curcumin in
Alzheimer's disease: Targeting neuroinflammation strategies
Phytotherapy Research | April 2024
Curcumin, a
polyphenolic compound derived from Curcuma longa, has
shown potential neuroprotective effects due to its
anti-inflammatory and antioxidant properties. An exhaustive
literature search was conducted, focusing on recent studies
within the last 10 years related to curcumin's impact on
neuroinflammation and its neuroprotective role in Alzheimer's
disease. The review methodology included sourcing articles from
specialized databases using specific medical subject headings
terms to ensure precision and relevance. Curcumin demonstrates
significant neuroprotective properties by modulating
neuroinflammatory pathways, scavenging reactive oxygen species,
and inhibiting the production of pro-inflammatory cytokines.
Curcumin emerges as a promising therapeutic adjunct in
Alzheimer's disease due to its multimodal neuroprotective
benefits.
Application and Potential Value of Curcumin in
Prostate Cancer: A Meta-Analysis Based on Animal Models
Frontiers in Pharmacology | April 2024
Curcumin is gaining
recognition as an agent for cancer chemoprevention and is
presently administered to humans. The findings of this
meta-analysis demonstrated that curcumin exhibited a superior
inhibitory effect on the volume of prostate cancer tumors in
mice compared to the control group. Additionally, curcumin
displayed a more effective inhibition of mice prostate cancer
tumor weight. Furthermore, in terms of tumor inhibition rate,
curcumin exhibited greater efficacy. Moreover, curcumin more
effectively inhibited PCNA mRNA and MMP2 mRNA. Curcumin
exhibited inhibitory properties towards prostate tumor growth
and demonstrated a beneficial effect on prostate cancer
treatment, thereby offering substantiation for further clinical
investigations.
The mechanistic role of curcumin on matrix
metalloproteinases in osteoarthritis
Fitoterapia | April 2024
Curcumin demonstrates significant
improvements in inflammation, pain and function scores. Curcumin
demonstrated significant inhibition of matrix metalloproteinases
linked to cartilage degradation in Osteoarthritis through
reduced activation of the nuclear factor kappa-B signaling
pathway via suppressing phosphorylation of Iκβa and p65 nuclear
translocation. Mechanistic evidence implicated matrix
metalloproteinases in Osteoarthritis by decreasing Type II
collagen, leading to cartilage damage. As a potential
nutritional intervention for Osteoarthritis, curcumin could
reduce inflammatory markers and improve pain and function
scores. Curcumin, a bioactive compound from Curcuma longa, has
been extensively studied for its anti-inflammatory properties
[19]. Curcumin inhibits NFκB activation, reducing IL-1, IL-6,
and TNF-α levels. In vitro studies have shown curcumin's ability
to modulate MMPs by inhibiting NFκB activation in both animal
and human chondrocytes [[13], [14], [15], [16], [17], [18]].
Human studies have reported significant improvements in
inflammatory markers with curcumin treatment. This systematic
literature review supports all three mechanistic hypotheses,
demonstrating curcumin could inhibit MMPs and therefore
osteoarthritis expression of cartilage damage. Curcumin was
demonstrated to inhibit MMP-1, −3 and − 13 linked to cartilage
degradation in osteoarthritis. Curcumin was also shown to
inhibit MMPs by suppressing activation of the NFκB signaling
pathway. This was observed by two means: curcumin's suppression
of Iκβa phosphorylation and inhibition of p65 nuclear
Protective Effects of Curcumin and Resveratrol on
Kidney Tissue on Cadmium- induced Oxidative Stress in Rats
Çanakkale Onsekiz Mart University | April 2024
Curcumin has a
wide spectrum of effects, including anti- inflammatory,
antioxidant, anticarcinogenic, antidiabetic, antiviral, and
neuroprotective effects. It facilitates the removal of many
reactive oxygen radicals, especially superoxide anions. In
addition, it has been reported to scavenge ROS, inhibit lipid
peroxidation, and protect cellular macromolecules from oxidative
damage.In recent studies, curcumin has attracted attention for
its potential antioxidant or anti-apoptotic properties. Curcumin
has many beneficial properties, including antioxidant and
anti-inflammatory actions (24-26). In this study, it was
observed that the TAC level increased and the MDA level and OSI
value decreased in the group administered curcumin with Cd. On
the basis of our results, we can say that curcumin may benefit
kidney tissue in cadmium-induced oxidative stress. As a result,
both resveratrol and curcumin support the defense system of
cells by scavenging free radicals that increase the oxidative
damage caused by Cd in the kidneys.
Effect
of curcumin on the expression of NOD2 receptor and
pro-inflammatory cytokines in fibroblast-like synoviocytes of
rheumatoid arthritis patients
Advances in
Rheumatology | April 2024
Studies have shown that curcumin
inhibits proliferation, migration, invasion, and Inflammation
and on the other hand increases the apoptosis of rheumatoid
arthritis FLSs. In this study, we aim to evaluate the effect of
curcumin, a natural antiinflammatory micronutrient, on the
expression of NOD2 and inflammatory cytokines. Administering
curcumin alone or in combination whit MDP can significantly
reduce mRNA expression levels of P65 and IL-6 in FLSs of both
groups. Moreover, in FLSs of rheumatoid arthritis patients, a
single curcumin treatment leads to a significant reduction in
NOD2 gene expression. This study provides preliminary in vitro
evidence of the potential benefits of curcumin as a nutritional
supplement for rheumatoid arthritis patients. Despite the
limitations of the study being an investigation of the FLSs of
rheumatoid arthritis patients, the results demonstrate that
curcumin has an anti-inflammatory effect on NOD2 and NF-κB
genes. These findings suggest that curcumin could be a promising
approach to relieve symptoms of rheumatoid arthritis.
Effects of curcumin supplementation on abdominal
surgical wound healing
ACTA Cirúrgica
Brasileira | April 2024
Numerous scientific studies have
demonstrated the anti-inflammatory and antioxidant properties of
curcumin, resulting in the reduction of free radicals and
optimization of wound healing. In the present study, oral
administration of curcumin via gavage demonstrated effectiveness
in reducing blood vessels compared to the control group.
Curcumin is a potential aid in wound contraction, as it
accelerates the phases of the healing process. Its
anti-inflammatory action is attributed to its ability to reduce
inflammatory pathways, such as interleukin (IL)-1, IL-6, and
transcription factors of protein I and nuclear factor kappa B (NfkB).
During the proliferative phase, curcumin is capable of
increasing reepithelialization speed, as well as granulation
tissue and the amount of type III collagen. Oral curcumin
supplementation was able to significantly reduce inflammatory
parameters in both preoperative phase and postoperative phase
(inflammatory infiltrate and blood vessel count) in abdominal
surgical wounds of Wistar rats.
Curcumin
intervention in hippocampal atrophy of diet-induced
Alzheimer-like deficits in insulin-resistant rats
Rwanda Medical Journal | April 2024
Curcumin is a prime
contender against pervasive diseases due to its efficacy,
accessibility, affordability, and safety. The findings
demonstrated that oral curcumin effectively corrected
hyperglycemia and reduced insulin resistance. The study further
revealed that insulin resistance was related to hippocampal
atrophy and related deficits in the assessed rat model. Curcumin
ameliorated these changes, reduced the aggregation of Aβ in the
hippocampus, and reversed impaired signaling of proteins PI3K,
AKT, and GSK-3β. The study's findings imply that oral curcumin
has potential therapeutic advantages against prevalent neuronal
death linked to abnormalities mimicking Alzheimer's disease and
insulin resistance. Hence, curcumin may benefit dementia
patients who also have insulin resistance.
Potential of Curcumin in the Management of Skin Diseases
Molecular Sciences | April 2024
Curcumin is a polyphenolic
molecule derived from the rhizoma of Curcuma longa L.
This compound has been used for centuries due to its
anti-inflammatory, antioxidant, and antimicrobial properties.
These make it ideal for preventing and treating skin
inflammation, premature skin ageing, psoriasis, and acne.
Additionally, it exhibits antiviral, antimutagenic, and
antifungal effects. Curcumin provides protection against skin
damage caused by prolonged exposure to UVB radiation. It reduces
wound healing times and improves collagen deposition. Moreover,
it increases fibroblast and vascular density in wounds. This
review summarizes the available information on the therapeutic
effect of curcumin in treating skin diseases. The results
suggest that curcumin may be an inexpensive, well-tolerated, and
effective agent for treating skin diseases. Curcumin has a broad
spectrum of biological potentialities, of which
anti-inflammatory and cardioprotective effects are most often
investigated. Turmeric, which is the main source of curcumin, is
known for its health-promoting properties since the ancient
times. There is an increasing number of studies that state that
curcumin can modulate phenomena associated with inflammatory,
infectious, and proliferative skin diseases. It is applied
for health-promoting purposes due to its multidirectional
action, of which its antioxidant activity is a very important
issue.
Curcumin inhibits the growth and invasion of gastric
cancer by regulating long noncoding RNA
World Journal of Gastrointestinal Oncology | April 2024
Curcumin, a natural anticancer agent, exhibits therapeutic
promise in gastric cancer. Its effects include promoting cell
apoptosis, curtailing tumor angiogenesis, and enhancing
sensitivity to radiation and chemotherapy. Curcumin exerts
anticancer effects by inhibiting cell cycle progression and
promoting apoptosis. Numerous studies have substantiated the
antitumor properties of curcumin, whether used in isolation or
in conjunction with other pharmaceutical agents. Research has
shown that curcumin can diminish H19 expression while augmenting
P53 in GC cells, thereby manifesting antiproliferative effects.
Our experimental findings corroborate that curcumin markedly
curtails the proliferation of GC cells, including AGS, MGC-803,
and BGC-823 cells. Curcumin has potential anticancer effects on
gastric cancer cells by regulating RNA.
Role of Curcumin in the Management of Rheumatoid
Arthritis and Psoriatic Arthritis
Psoriasis
- Recent Advances in Diagnosis and Treatment | April 2024
Curcumin, the primary active component within Curcuma longa
(turmeric), has been demonstrated to be helpful in treating
rheumatoid arthritis and psoriatic arthritis, with effectiveness
attributed to its mode of activity. The antiarthritic effect of
curcumin has been investigated in patients with rheumatoid
arthritis. In one study, 45 participants was randomly allocated
to one of three groups: treatment with 500 mg of curcumin,
treatment with 50 mg of diclofenac sodium, or a combination of
the two. The Disease Activity Scores of patients across all
three groups showed significant statistical improvement.
However, the curcumin group showed superior results and had the
highest percentage of recovery. According to the American
College of Rheumatology score, the curcumin group also exhibited
the greatest reduction in pain and joint edema. According to
various literature surveys and evidence, it can be concluded
that curcumin is a safe and effective therapeutic option for
managing rheumatoid arthritis and psoriatic arthritis compared
to synthetic medications. Turmeric regulates inflammation,
cell development, and apoptosis, making it suitable for both
prevention and treatment of various ailments due to its
antioxidant and anti-inflam- matory applications, along with its
high safety profile, the bulk of which is attributed to
curcumin. Curcumin interacts with a wide variety of molecular
domains, making it a pleiotropic chemical. According to in vitro
and in vivo research, curcumin is a promising curative drug for
various chronic illnesses, including pancreatitis, inflammatory
bowel disease, chronic anterior uveitis, and certain
malignancies. Curcumin’s physiological actions or molecular
processes have been explored in numerous cell and animal
investigations, and research is ongoing. Curcumin has attracted
attention as a possible rheumatoid arthritis treatment due to
its antioxidant properties and regulatory role of associated
inflammatory agents. Curcumin, an antioxidant,
anti-inflammatory, and immune-modulatory agent, is found in
turmeric. It has been found to assist with arthritis and joint
pain. Given that persistent inflammation is a key feature of
arthritis, it is important to note that curcumin has been
reported to be beneficial for both inflammatory bowel disease
and irritable bowel syndrome. Additionally, curcumin may
potentially help reduce neuroinflammation associated with
Alzheimer’s disease. Recent studies suggest that curcuminoids,
when combined with black pepper, may help alleviate the painful
joint symptoms associated with RA, PsA, or OA. Curcumin can help
the immune response by reducing inflammation, which can help to
alleviate joint discomfort, increase mobility, and restore
functioning in troublesome regions. Curcumin’s anti-inflammatory
properties have been proven in several trials, including
investigations of back pain, muscular discomfort, and
inflammation associated with asthma and allergies. Curcumin has
been demonstrated to be safe and effective in treating
rheumatoid arthritis and psoriatic arthritis. Curcumin has
proven beneficial in effectively treating both diseases without
adverse side effects, potentially leading to symptom improvement
in both conditions.
Curcumin is comparable to metformin for the treatment of
PCOS in rats: a preclinical study
Pharmacia
| April 2024
Curcumin, the active ingredient in turmeric
(Curcuma longa Zingiberaceae) is a yellow polyphenol with a wide
range of pharmacological activities such as antioxidant,
anti-inflammatory, anti-tumor, neuroprotective and
cardioprotective properties. Weight was significantly reduced in
the curcumin, metformin and curcumin and metformin combination
groups. Curcumin is therapeutically effective for PCOS and is
comparable to metformin. Curcumin is a major herbal constituent
of turmeric (Nelson et al. 2017), it had been used in herbal
medicine for ages, partially for its anti-inflammatory effects (Mahdizadeh
et al. 2015). Curcumin, that golden concoction, is used for
almost every ailment, acute and chronic (Amalraj et al. 2017;
Kunnumakkara et al. 2017). From cancer to obesity, no health
problem has not been presumed to profit from curcumin, mainly
due to curcumin’s antineoplastic, antioxidant, antimicrobial,
and anti-inflammatory qualities (Kocaadam and Şanlier 2017; Li
et al. 2018). Increasingly available evidence is accumulating
regarding curcumin potential efficacy for pulmonary diseases
(Lelli et al. 2017). Curcumin has also shown the potential to be
effective for skin diseases such as psoriasis, atopic
dermatitis, and wound care (Vollono et al. 2019). Additionally,
curcumin is non-toxic and safe for humans’ consumption (Soleimani
et al. 2018). Curcumin anti-inflammatory properties make it
potentially useful for many disorders and ailments whose
pathophysiological basis implicates inflammation. Type II
diabetes (T2D) a prevalent modern world disorder, is also
potentially responsive to curcumin in pre- and clinical studies
(Pivari et al. 2019). Endometriosis, a gynecological disorder
marked by inflammation, appears to benefit from the
anti-inflammatory effects of curcumin (Arablou and Kolahdouz-Mohammadi
2018). Curcumin effects were expected as it had been shown to be
effective for many disorders including gynecological disorders (Arablou
and Kolahdouz-Mohammadi 2018). One possible mechanism for
curcumin effects on PCOS signs might be its antioxidant powers
as oxidative stress has a role in pathogenesis of PCOS (Ryu et
al. 2019). Curcumin anti-inflammatory properties also most
probably contribute to its effectiveness in disorders such as
PCOS (Salehi et al. 2019). Also, the effect of curcumin could be
through its beneficial effects on glucose tolerance and lipid
profile which when improved can also improve symptoms of PCOS.
Curcumin improved insulin sensitivity and lipid profiles in
rats’ models of type 2 diabetes (Francesca Pivari, Alessandra
Mingione 2019). This study did not attempt to examine the exact
mechanism of curcumin effects in this model of PCOS. Thus, we
cannot be certain about the mechanism(s) of curcumin in
improving symptoms of PCOS in this study.
Network pharmacology and molecular docking reveal
the mechanisms of curcumin activity against esophageal squamous
cell carcinoma
Frontiers in Pharmacology |
April 2024
Curcumin displays anti-cancer activity against
various cancers. Numerous lines of evidence indicate that
curcumin has a potential in the management of various forms of
cancer, including prostate cancer (Termini et al., 2020),
colorectal cancer (Pricci et al., 2020), breast cancer (Deng et
al., 2022), endometrial carcinoma (Zhang et al., 2019) and
non-small cell lung cancer (Xie et al., 2022). Curcumin has
potential anti-ESCC activity. For instance, curcumin can induce
apoptosis and enhance the sensitivity of fluorouracil ESCC cell
death by suppressing the NF-κB signaling pathway (Tian et al.,
2012). According to Deng and his colleagues, the combination of
curcumin and docetaxel triggers cell death and self-degradation
in ESCC cells through the PI3K/AKT/mTOR signaling pathway (Deng
et al., 2021). In ESCC cells, curcumin inhibits STAT3-mediated
signaling and induces apoptosis and growth arrest (Liu et al.,
2018). Curcumin has been widely used in cancer treatment (Bar-Sela
et al., 2010; Zoi et al., 2021). A recent study found that
curcumin has the ability to regulate the
circNRIP1/miR-532-3p/AKT pathway, resulting in the suppression
of ESCC (Luo et al., 2023). Furthermore, curcumin induces
apoptosis (Liu et al., 2018; Deng et al., 2021) and suppresses
esophageal squamous cell carcinoma cell proliferation (Hosseini
et al., 2018), and blocks the growth of primary esophageal
squamous cell carcinoma-derived xenografts in vitro (Liu et al.,
2018). Based on the findings from the GO and KEGG analyses,
curcumin has the potential to address esophageal squamous cell
carcinoma by modulating pathways related to cell cycle,
programmed cell death, and cellular aging. Our experiments
showed that curcumin induced a pause in the cell cycle at the
G2/M and S stages. CCK8 experiments and clone formation assays
indicated that curcumin significantly inhibited esophageal
squamous cell carcinoma cell proliferation. Furthermore,
Transwell invasion assays demonstrated that curcumin inhibited
esophageal squamous cell carcinoma cell invasion and SA-β-gal
measurement indicated that curcumin can induce esophageal
squamous cell carcinoma cell senescence. Western blot results
showed that curcumin may treat esophageal squamous cell
carcinoma by inhibiting CDK2/RB pathway. 5 Conclusion In
summary, our findings from network pharmacology, molecular
docking, and in vitro experimental confirm that curcumin
possesses the capacity to inhibit the proliferation and invasion
of esophageal squamous cell carcinoma cells. This is achieved
through the induction of cell cycle arrest, regulation of FoxO,
cell senescence, IL-17, and many cancer-related signaling
pathways. Curcumin may exert anti-ESCC effects by interacting
with CHEK1, TOP2A, CDK2, AURKA, CDK6, DHFR, EGFR, STAT3, PPARG,
and SERPINE1CUR arrested ESCC cells at the G2/M and S phases, as
shown by flow cytometry. Colony formation and CCK8 assays showed
that curcumin can inhibit the proliferative ability of
esophageal squamous cell carcinoma cells. The Transwell invasion
results validated that curcumin can significantly inhibit the
invasion rates of esophageal squamous cell carcinoma cells.
Collectively, these findings indicate that curcumin exhibits
pharmacological effects on multiple targets and pathways in
esophageal squamous cell carcinoma.
The Effects of Curcumin Plus Piperine Co-administration on
Inflammation and Oxidative Stress: A Systematic Review and
Meta-analysis of Randomized Controlled Trials
Current Medicinal Chemistry | April 2024
Results indicated
that curcumin plus piperine administration could effectively
reduce oxidative stress and inflammation. The beneficial effects
of curcumin against various chronic disorders have been shown in
the last few decades. Piperine has been used in scientific
evaluations as an effective compound to increase the
bioavailability of curcumin. According to the current
meta-analysis, curcumin plus piperine administration showed a
significantly increased SOD activity and GSH levels while
significantly decreased MDA concentrations. In addition, our
study revealed that curcumin plus piperine significantly
decreased TNF-α and IL-6 concentrations.
NF-κB pathway as a molecular target for curcumin in
diabetes mellitus treatment: Focusing on oxidative stress and
inflammation
Authorea | April 2024
This
study extensively explores the prospective therapeutic benefits
of curcumin, a bioactive compound known for its antioxidant,
anti-inflammatory, and hypoglycemic properties, in addressing
diabetic complications, predominantly via the modulation of the
NF-κB pathway. The findings reveal that curcumin administration
effectively lowered blood glucose elevation, reinstated
diminished serum insulin levels, and enhanced body weight in
Streptozotocin -induced diabetic rats. Curcumin exerts its
beneficial effects in management of diabetic complications
through regulation of signaling pathways, such as CaMKII,
PPAR-γ, NF-κB, and TGF-β1. Moreover, curcumin reversed the
heightened expression of inflammatory cytokines (TNF-α, IL-1β,
IL-6) and chemokines like MCP-1 in diabetic specimens,
vindicating its anti-inflammatory potency in counteracting
hyperglycemia-induced alterations. Curcumin diminishes oxidative
stress, avert structural kidney damage linked to diabetic
nephropathy, and suppress NF-κB activity. Furthermore, curcumin
exhibited a protective effect against diabetic cardiomyopathy,
lung injury, and diabetic gastroparesis. Conclusively, the study
posits that curcumin could potentially offer therapeutic
benefits in relieving diabetic complications through its
influence on the NF-κB pathway. Curcumin has shown potency
against numerous chronic conditions like Alzheimer’s disease,
T2DM, rheumatoid arthritis, and metabolic syndrome. It possesses
a broad spectrum of therapeutic properties, including
antineoplastic, antimicrobial, anti-carcinogenic,
anti-mutagenic, anti-aging, anti-inflammatory,
anti-proliferative, anti-amyloid, and anti-hypercholesterolemic
effects. Mechanistically, curcumin inhibits the initiation of
the radical-sensitive transcription factor NF-κB, reduces
cytokine production, and impedes vital cellular survival
processes. It also inhibits STAT proteins and NF-κB-DNA binding,
diminishing the expression of pro-inflammatory molecules MMP-9,
MMP-3, and cytokines like TNF-α, IL-1, and IL-8. Furthermore,
curcumin attaches to the COX-2 protein, curtailing COX-2
expression and the synthesis of prostaglandins and thromboxanes
[61]. Clinical trials have consistently affirmed curcumin’s
assurance, acceptability, and effectiveness in addressing
diverse chronic disorders in humans. These trials reported no
toxicity when curcumin was given by mouth at a daily dose of 6 g
for a duration of 4 to 7 weeks. The study underscores curcumin’s
potential as a therapeutic agent in mitigating diabetes mellitus
complications, primarily through modulating the NF-κB pathway.
By demonstrating anti-inflammatory, antioxidative, and
anti-fibrotic properties, curcumin has been effective in
managing multiple diabetic conditions, including nephropathy,
cardiomyopathy, and gastroparesis. The alteration of critical
signaling pathways such as CaMKII, PPAR-γ, NF-κB, and TGF-β1 is
pivotal in curcumin’s beneficial effects. Additionally, its
capacity to diminish OS, curb inflammation, and enhance vascular
functionality vindicate curcumin as a promising candidate
molecule for management of diabetes mellitus complication. These
findings highlight the necessity to further explore the
potential of curcumin as a supplementary treatment option in
management of diabetes mellitus complications.
Regulatory effects of curcumin on nitric oxide
signaling in the cardiovascular system
Nitric Oxide | April 2024
Curcumin improves cardiovascular
function, acting as an anti-inflammatory, antioxidant, and
increasing bioavailability of nitric oxide. The major component
of Curcuma longa, curcumin, has long been utilized in
traditional medicine to treat various illnesses, especially
cardiovascular diseases. Curcumin's pharmacological effects have
been well studied. It works by lowering the generation of ROS,
restricting the entrance of potassium, suppressing NF-κB,
extracellularly regulated protein kinases (ERK) 1/2 and c-Jun
N-terminal kinase (JNK), and preventing the activity of
apoptosis-associated speck-like protein (ASC). Furthermore,
curcumin stimulates the sirtuin1 (SIRT1)-forkhead box
transcription factor O1 (FOXO1) axis, which decreases cardiac
oxidative stress levels and debilitates myocardial apoptosis. By
the SIRT1/adenovirus E1B 19 kDa-interacting protein 3
(BNIP3)/B-cell lymphoma-2 (Bcl-2)/Beclin-1 autophagy axis,
curcumin may also preserve cardiomyocytes. Curcumin enhances
left ventricular function in pressure-overloaded rabbits by
inhibiting collagen remodeling and reducing the expression of
TNF-a and MMPs in the myocardium. Tharaux et al. demonstrated
that curcumin inhibits fibrosis in the aorta and renal arteries,
preserving vessel wall elasticity. Curcumin was found to exert
these effects by inhibiting the renin-angiotensin
system-transforming growth factor β1 (TGF-β1) collagen axis.
Curcumin improves cardiovascular function as well as having
important pleiotropic effects, such as anti-inflammatory and
antioxidant, through its ability to increase the bioavailability
of nitric-oxide and to positively impact nitric-oxide related
signaling pathways.
Protective Effect of Curcumin on D-Galactose-Induced Senescence
and Oxidative Stress in LLC-PK1 and HK-2 Cells
Antioxidants | April 2024
Curcumin has been reported to exert
antioxidant, anti-inflammatory, antimutagenic, antimicrobial,
hypoglycemic, and anticancer effects. Moreover, its safety,
tolerability, and non-toxicity have been reported in clinical
trials. Regarding its antioxidant effects, curcumin is a
bifunctional antioxidant. It has two phenol groups which confer
a high hydrogen-donating activity and directly scavenges ROS.
Furthermore, this curcumin polyphenol increases the activity of
antioxidant enzymes such as CAT, SOD, glutathione peroxidase,
and heme oxygenase-1 through the activation of nuclear factor
erythroid 2-related factor (Nrf2) that induces the expression of
genes coding for elements of the antioxidant system.
Furthermore, curcumin has also been considered an anti-aging and
senolytic agent in different in vitro and in vivo models.
Curcumin, through its antioxidant properties, decreased DNA
damage and reduced the number of senescent cells, preventing the
decrease in lamin B1 in LLC-PK1 cells. It has been observed that
the expression of lamin B1 is decreased by oxidative stress and
is related to the senescent phenotype. Our results show that
curcumin prevents changes induced by D-galactose in different
senescence markers, notably β-galactosidase activity (decrease
of 20% in LLC-PK1 cells) and PCNA (decrease of 60% in HK-2
cells). Moreover, it decreases oxidative stress (ROS reduction
of 20% in both cell lines) and oxidative damage (8-OHdG
reduction of 25% in LLC-PK1 cells) caused by D-galactose
treatment. Therefore, curcumin has a moderate protective effect
on D-galactose-induced senescence and oxidative stress in
LLC-PK1 and HK-2 cells and could be further considered as a
possible therapeutic agent. Curcumin treatment decreased by 20%
the number of cells positive for senescence-associated
(SA)-β-D-galactosidase staining and by 25% the expression of
8-hydroxy-2′-deoxyguanosine (8-OHdG) and increased by 40% lamin
B1 expression. In HK-2 cells, curcumin treatment increased by
60% the expression of proliferating cell nuclear antigen (PCNA,
50% Klotho levels, and 175% catalase activity. In both cell
lines, this curcumin antioxidant decreased the production of ROS
(20% decrease for LLC-PK1 and 10 to 20% for HK-2). These data
suggest that curcumin treatment has a moderate protective effect
on D-galactose-induced senescence in LLC-PK1 and HK-2 cells.
The Modulatory Effects of Curcumin on the Gut Microbiota: A
Potential Strategy for Disease Treatment and Health Promotion
Microorganisms | March 2024
In addition to its antioxidant
properties, curcumin exhibits various other biological
activities, such as anti-inflammatory effects, the modulation of
lipid metabolism, and the modulation of the immune system. These
activities contribute to its potential as an anticancer,
antitumor, and antithrombotic agent. Furthermore, curcumin is
known to interact with various cellular and molecular targets,
including growth factors, chemokines, transcription factors, and
cell adhesion molecules, further enhancing its therapeutic
potential. The contradiction with the low bioavailability of
curcumin and its diverse pharmacological activities could be
resolved by considering the interactions of curcumin with the
gut microbiota. Since this discovery, a large number of clinical
trials started to explore the therapeutic potential of curcumin
for a variety of human diseases, namely cancer, cardiovascular
diseases, neurodegenerative diseases, and inflammation. The
latest research indicates elevated levels of curcumin in the
gastrointestinal system after the oral intake of curcumin. It
has been proposed that curcumin interacts dynamically with the
intestinal microbiota, modulating the composition and activity
and exerting a potential therapeutic effect on diseases caused
by the dysbiosis of the intestinal flora. It has been found that
oral curcumin supplementation is able to target improvements in
gut barrier function and that higher levels of curcumin residues
have been found in the gut after oral curcumin administration,
emphasizing the possible positive effects of curcumin on the gut
microbiota. The important biological activities of curcumin,
such as anti-inflammatory, antioxidant, anti-obesity, and
anticancer, have long been demonstrated, and it is able to fully
utilize its pharmacological effects through various molecular
targets. The anti-inflammatory effect of curcumin is mainly
achieved by inhibiting the NF-κB, reducing the release of a
variety of pro-inflammatory factors, accompanied by the
scavenging of free radicals and the downregulation of ROS
generation, thereby reducing oxidative stress and exerting its
antioxidant effects. Therefore, curcumin can improve the
occurrence and development of atherosclerosis and inflammatory
bowel disease. In addition, curcumin maintains intestinal
barrier function by regulating multiple signaling pathways to
prevent damage from dietary factors or endogenous injury.
Secondly, curcumin can regulate lipid metabolism and production
through MPAK signaling, thus achieving the goals of treatments
for obesity.
Curcumin as a regulator of Th17 cells: Unveiling the
mechanisms
Food Chemistry Oxford | March
2024
Curcumin exhibits anticancer, anti-inflammatory, and
antioxidant characteristics (Chatterjee and Pandey, 2011,
Marjaneh et al., 2018, Zahedi et al., 2023, Mohajeri and
Sahebkar, 2018). In the past two decades, studies have found
that curcumin has immunomodulatory effects, regulating the
activity of immune cells. Recent reports have shown that
curcumin can reduce the proliferation of alloreactive T cells
and significantly suppress the differentiation of Th17 cells
(Park et al., 2013). Curcumin also suppresses the production of
pro-inflammatory cytokines, including IL-17 and TNF-α, in Th17
cells, thereby reducing the expression of inflammatory cells,
particularly neutrophils, and resulting in decreased
inflammation and infiltration (Mohammadian Haftcheshmeh et al.,
2021). Curcumin demonstrates promise in regulating T cell
subsets associated with inflammatory diseases. Xiao et al. (Xiao
et al., 2022) reported that curcumin downregulated
pro-inflammatory Th17 cells (CD4 + CCR6 + Th17, CD4 + IL-17A +
Th17, IL-17A, BATF, C-Maf, RORγt) and upregulated
anti-inflammatory Treg cells (CD4 + Foxp3 + Treg, CD4 + IL-10 +
Treg, IL-10, Foxp3, Eomes) in mice with diabetic colitis,
suggesting its ability to restore Th17/Treg balance. Human
studies on curcumin's systemic bioavailability, metabolites, and
pharmacokinetics have primarily focused on patients with cancer.
Garcea et al. (Garcea et al., 2005) found that patients with
different stages of colorectal cancer who took 3.6 g of curcumin
for 7 days had pharmacologically effective concentrations of
curcumin in both normal and colorectal tissue (Menozzi et al.,
2009). Curcumin has several roles in the regulation of enzymes
involved in inflammation, adhesion molecules, transcription
factors, protein kinases, cytokines, and redox balance. It
possesses various pharmacological functions, including
antimicrobial, antitumor, anti-inflammatory, antiviral,
antioxidant, and antifungal properties (Howells et al., 2021,
Heidari et al., 2023, Bagheri et al., 2020, Cicero et al., 2020,
Keihanian et al., 2018, Khayatan et al., 2022, Mokhtari-Zaer et
al., 2018, Panahi et al., 2019, Sahebkar, 2010, Sahebkar, 2014,
Iranshahi et al., 2010, Mohammadi et al., 2019, Panahi et al.,
2012). It also plays a protective role against autoimmune
diseases. Several studies have demonstrated that curcumin can
enhance the antitumor effects of chemotherapeutics, including
paclitaxel, against various cancers while also reducing the side
effects of conventional chemotherapies (Ashrafizadeh et al.,
2020). Curcumin acts as an active scavenger of reactive oxygen
species (ROS) and reactive nitrogen species. Moreover, it
increases the synthesis of glutathione (GSH), a crucial
intracellular antioxidant (Menon and Sudheer, 2007, Zheng et
al., 2017). Curcumin also plays a role in protecting against
catalase activities and reducing the levels of glutathione
S-transferases (GST), superoxide dismutase (SOD), and
glutathione peroxidase (GPx) (Arshad et al., 2017). Because
oxidative stress is closely related to inflammation, curcumin
indirectly inhibits pro-inflammatory reactions in the immune
system (He et al., 2015). Notably, curcumin is a potent
immunomodulator, with strong interactions with different types
of immune cells, including neutrophils, T cells, mast cells,
epithelial cells, eosinophils, and basophils, resulting in the
regulation of pathological immune system reactions (Panahi et
al., 2015, Mohammadi et al., 2022, Rahimi et al., 2019). The
immunomodulatory effects of curcumin, which confer protection
against immune-mediated diseases, are attributed to its ability
to interact with cells of the immune system (Gao et al., 2004,
Gautam et al., 2007, Momtazi-Borojeni et al., 2018). Curcumin
can influence both the innate and adaptive immune reactions (Haftcheshmeh
et al., 2022, Gao et al., 2004). Curcumin's immunomodulatory
properties arise from its ability to modulate the expression of
numerous pro-inflammatory chemokines and cytokines, including
interleukin (IL)-1β, tumor necrosis factor (TNF)-α, IL-6, and
monocyte chemoattractant protein-1 (MCP-1) (Haftcheshmeh et al.,
2022, Gao et al., 2004, Momtazi-Borojeni et al., 2018, Panahi et
al., 2016). Curcumin, an active compound found in turmeric,
exhibits potent immunomodulatory effects, making it a promising
therapeutic agent for various inflammatory and autoimmune
disorders. Recent studies have focused on the ability of
curcumin to modulate Th17-mediated immune responses, which play
a critical role in the development of several autoimmune
diseases. Curcumin has been shown to inhibit the proliferation
and differentiation of Th17 cells and reduce the production of
specific pro-inflammatory cytokines such as IL-17, IL-6, and
TNF-α. Additionally, curcumin has been found to increase the
production of Treg cells, which suppress excessive immune
responses and maintain immune homeostasis. Overall, curcumin's
ability to target the Th17 axis opens exciting avenues for
developing novel therapeutic strategies for autoimmune and
inflammatory diseases.
Phytocompounds-based therapeutic approach: Investigating
curcumin and green tea extracts on MCF-7 breast cancer cell line
Journal of Genetic Engineering and
Biotechnology | March 2024
Results showed the anticancer and
cytotoxic properties of curcumin against MCF-7 cells, with a
relatively higher level of released LDH. The curcumin extract
downregulated the oncogenic Raf-1, suppressed the Telomerase and
upregulated the TNF-α and IL-8 genes. Results from the ELISA
showed a notable increase in IL-8 and TNF-α cytokines levels
after curcumin treatment, which culminated after 72 h. Among
both extracts, only curcumin effectively modulated the
understudy molecular targets, achieving multi-targeting
anticancer activity against MCF-7 cells. Moreover, the applied
dosage significantly increased levels of the proinflammatory
cytokines, which represent a component of the
cytokines-targeting-based therapeutic strategy.
Cytotoxic evaluation of curcumin and quercetin in
MCF-7 cell lines
World Journal of Biology
Pharmacy and Health Sciences | March 2024
Curcumin, a
naturally occurring compound found in turmeric, has been
extensively studied for its potential anticancer properties,
including its effects on MCF-7 cell lines. MCF-7 cells are
commonly used as a model for studying breast Anti-Inflammatory
Effects: Both curcumin and quercetin possesses anti-inflammatory
properties, and chronic inflammation is often associated with
cancer development. By reducing inflammation, curcumin may
indirectly contribute to the cytotoxic effect on cancer cells.
In this study, the cytotoxic potential of a curcumin was found
to be greater than the cytotoxic potential of quercetin in the
individual testing, however the phyto nutrient combination &
natural extracts of both curcumin and quercetin shows the better
cytotoxic potential in the MCF7 Breast cancer cell line. The
combination of both the phytoconstituents shows an effective
synergistic effect that can elicit a powerful cytotoxic response
on the breast cancer cells. The synergistic effects observed in
the combination of curcumin and quercetin on MCF-7 cells suggest
that the compounds may act through complementary mechanisms,
enhancing their individual anticancer activities.
Impact of Curcumin on Aging: Manifestations and
Limitations
Curcumin and Neurodegenerative
Diseases | March 2024
Due to its potential for treating
cancer and Alzheimer’s disease, turmeric, which contains
curcumin, has gained considerable attention in recent years. The
primary reason why curcumin is effective is its
anti-inflammatory properties. Age-related redox imbalance
results in overexpression of reactive oxygen species levels.
Curcumin reduces oxidative stress associated with cellular
senescence and can benefit aging individuals.
Exploring the molecular mechanism of action of
curcumin for the treatment of diabetic retinopathy, using
network pharmacology, molecular docking, and molecular dynamics
simulation
Integrative Medicine Discovery |
March 2024
A wide range of pharmacological properties have
been attributed to curcumin, including antioxidant,
anti-inflammatory, antimutagenic, etc. Researchers found that
curcumin had a beneficial effect on the expression of vascular
endothelial growth factor (VEGF), tumor necrosis factor-alpha
(TNF-α), and pro-inflammatory cytokines in the retina of
diabetic rats. In addition, curcumin has also been demonstrated
to have the capability of slowing down or even reversing the
progression of certain fundus diseases, making it a new option
for the treatment of retinal diseases. As a result of this
study, curcumin may exert a role in the treatment of diabetic
retinopathy through multi-target and multi-pathway regulation,
which indicates a possible direction of future research. In
summary, through network pharmacology, molecular docking, and MD
simulations, the present study investigated the potential
mechanisms of curcumin in the treatment of diabetic retinopathy.
Following its introduction into the body, curcumin has multiple
targets and pathways for treating diabetic retinopathy. Several
targets were shown to be used by curcumin to exert anti-diabetic
retinopathy effects, including AKT1, IL-1B, IL-6, ALB, and
TNF-α, and regulating several signaling pathways such as
PI3K-Akt signaling pathway, lipid and atherosclerosis signaling
pathway, and AGE-RAGE signaling pathway in diabetic
complications.
Curcuma longa: A Natural Ally in Alzheimer’s Disease
Management
Curcumin and Neurodegenerative
Diseases | March 2024
Curcuma longa, commonly known
as turmeric or saffron, contains a compound called curcumin that
possesses potential biopharmacological activity. Curcumin is
considered safe by the American Food and Drug Administration
(FDA) and does not exhibit any side effects when consumed in
moderate amounts. Numerous studies have been conducted on
curcumin due to its therapeutic potential in various diseases,
particularly in neurodegenerative disorders such as Alzheimer’s
disease. Research indicates that this substance holds remarkable
potential as an effective and safe treatment.
Curcumin activates the JNK signaling pathway to
promote ferroptosis in colon cancer cells
Chemical Biology & Drug Design | March 2024
Recent evidence
has proved that curcumin as a natural polyphenol has anticancer
and anti-proliferative effects in cancer cells. Curcumin
suppressed SW-480 cancer cells viability in dose-dependent
manner. Cell treatment with curcumin led to accumulation of ROS
and iron within cells and increase in the intracellular levels
of lipid peroxidation. In addition, curcumin modulated the mRNA
and protein expression levels of ferroptosis-related proteins
including ACSL4, GPx4 and FTH1 and suppression of JNK signaling.
Curcumin may exhibit its anticancer effect on colorectal cancer
by downregulating JNK signaling to induce ferroptosis in SW-480
cells.
Longevity and anti-aging effects of curcumin
supplementation
GeroScience | March 2024
Curcumin demonstrated a positive impact on slowing down the
aging process by postponing age-related changes. Curcumin may
have anti-aging properties by changing levels of proteins
involved in the aging process, such as sirtuins and AMPK, and
inhibiting pro-aging proteins, such as NF-κB and mTOR. In
clinical research, this herbal compound has been extensively
examined in terms of safety, efficacy, and pharmacokinetics.
There are numerous effects of curcumin on mechanisms related to
aging and human diseases.
Curcuminoids with Antineurodegenerative Properties:
Current Trends and Future Perspectives
Curcumin and Neurodegenerative Diseases | March 2024
Curcuminoids are bioactive compounds found in Curcuma longa
Linn. (Zingiberaceae), a species commonly known as turmeric or
turmeric root, widely used in traditional medicine for
centuries. Recent research has demonstrated the potential of
these metabolites as antineurodegenerative agents, substances
that can help prevent or treat neurodegenerative diseases such
as Alzheimer’s disease, Parkinson’s disease, and multiple
sclerosis. Studies have focused on understanding the molecular
mechanisms by which curcuminoids exert their
antineurodegenerative properties, as well as developing more
effective and bioaccessible formulations of these compounds to
improve their bioavailability and ability to cross the
blood–brain barrier. Additionally, research has explored the
combination of curcumin with other substances or therapies, such
as combining them with other natural compounds, conventional
drugs, or non-pharmacological therapies like deep brain
stimulation.
Curcumin protects mice with myasthenia gravis by
regulating the gut microbiota, short-chain fatty acids, and the
Th17/Treg balance
Heliyon | March 2024
Curcumin modified the gut microbiota composition, increased
microbial diversity, and, in particular, reduced
endotoxin-producing Proteobacteria and Desulfovibrio levels in
T-AChR-induced gut dysbiosis. Moreover, we found that curcumin
significantly increased fecal butyrate levels in mice with T-AChR-induced
gut dysbiosis. In addition, curcumin repressed the increased
levels of lipopolysaccharide (LPS), zonulin, and FD4 in plasma.
Curcumin enhanced Occludin expression in the colons of MG mice
induced with T-AChR, indicating dramatically alleviated gut
permeability. Furthermore, curcumin treatment corrected T-AChR-induced
imbalances in Th17/Treg cells. Curcumin modifies gut microbiota
composition and increases the abundance of SCFA-producing
bacteria. Curcumin reduced FD4 concentrations as well as LPS and
zonulin levels in serum. Curcumin supplementation
increased the level of butyrate and regulated the TH17/Treg
balance. Curcumin is an effective constituent of the
traditional Asian medicine turmeric with antioxidant,
anti-inflammatory and neuroprotective properties. Of note,
curcumin has been recently proposed for the management of
neurological diseases by regulating the gut microbiota and
improving gut barrier function. It was reported that curcumin
could considerably alter the ratio of pathogenic and beneficial
bacteria by reducing the relative abundance of genera including
Prevotellaceae, Coriobacterales, Enterobacteria, and
Enterococci while increasing the relative abundance of
favorable genera including Bifidobacteria and
Lactobacilli. Hence, curcumin seems to have promising
clinical applications. We found that curcumin alleviated the
clinical symptoms, corrected the microbiota imbalance, increased
SCFA-producing bacteria, reduced intestinal permeability, and
regulated the Th17/Treg balance in T-AChR-induced MG mice.
Considering the protective effect of curcumin on the gut
microbiota and the regulation of immune responses by SCFAs, we
evaluated the concentrations of SCFAs in the feces by using
GC‒MS. In the T-AChR-induced EAMG group, fecal acetic acid and
butyric acid concentrations were decreased, whereas curcumin
administration reversed this reduction. These changes may be
directly correlated to the abundance of the genera
Oscillospira, Akkermansia, and Allobaculum.
Curcumin has been proven to be neuroprotective and
anti-inflammatory in both humans and animals; furthermore,
curcumin is used to treat Huntington's disease, Alzheimer's
disease, and cerebral ischemia.According to our study, curcumin
alleviated the clinical symptoms of MG mice, which was
consistent with previous reports. In addition, curcumin altered
the gut microbiota composition, increased SCFA-producing
bacteria, reduced intestinal permeability, and regulated the
Th17/Treg balance of T-AChR-induced MG mice. Curcumin and
its metabolites have been demonstrated to affect the gut
microbiota. Our findings have revealed that curcumin could
restore the original gut microbial composition in T-AChR-induced
MG mice and increase the abundance of a few important bacterial
species. However, the disorders of the gut microbiota during
EAMG were restored after treatment with curcumin, which mainly
manifested as an increase in gut microbial diversity and
richness. Consistently, curcumin increased the frequency of CD4+
CD25+ Foxp3+ Tregs and decreased the frequency of CD4+ IL-17A+
Th17 cells in spleens and inguinal lymph nodes. This shows that
curcumin has the ability to restore the imbalance of Th17/Treg
cells in T-AChR-induced MG mice. As a result, the host's immune
system contributes to the beneficial effects.
Curcumin Promotes Diabetic Foot Ulcer Wound Healing
by Inhibiting miR-152-3p and Activating the FBN1/TGF-β Pathway
Molecular Biotechnology | March 2024
Curcumin has been used
as a traditional remedy for inflammation and wound healing, and
it works at diverse stages, such as inflammation, maturation,
and proliferation, thereby accelerating the entire process of
wound healing. Studies have shown that curcumin treatment can
cause fibroblasts to infiltrate into the wound site and
accelerate wound healing. In addition, curcumin has been found
to be beneficial for diabetic foot ulcer and may be a
potential candidate for treatment. Curcumin inhibited the
apoptosis of fibroblasts, promoted their migration ability, and
alleviated the damage of fibroblasts stimulated by HG.
Furthermore, curcumin treatment promoted angiogenesis and
accelerated wound healing in diabetic foot ulcer rats.
This suggests that curcumin plays an important role in the
process of alleviating diabetic foot ulcer. According to
previous studies, there are many miRNAs involved in the
treatment of different diseases by curcumin. Studies have shown
that curcumin can attenuate vascular calcification through the
exosomal miR-4b-92p/KLF3 axis, and curcumin therapy protects
PC12 cells from high glucose-induced inflammatory responses.
Curcumin alleviates the progression of diabetic foot ulcer
by inhibiting miR-152-3p. In summary, curcumin activates
the FBN1/TGF-β pathway by inhibiting miR-152-3p, thereby
inhibiting HG-induced fibroblast apoptosis, promoting fibroblast
proliferation and migration, alleviating HG-induced fibroblast
damage, and promoting angiogenesis in diabetic foot ulcer
rats, thereby accelerating wound healing in diabetic foot
ulcer rats. This provides a new theoretical basis for curcumin
treatment of DFU and may become a potential therapeutic target
for diabetic foot ulcer.
Effects of Turmeric (Curcuma longa) on the Gut-Brain
Axis
Curcumin and Neurodegenerative
Diseases | March 2024
Turmeric is a spice widely known for
its medicinal properties, including its effects on gut and
neurobehavioral health. The results showed that curcumin is the
most studied bioactive compound in turmeric. In vitro and in
vivo studies with animal models have demonstrated antioxidant,
anti-inflammatory, antiviral, and anticancer properties of
turmeric and its isolated compounds. Some studies have shown the
effects of this spice against gastrointestinal diseases, on the
balance of the intestinal microbiota, and neurobehavioral
parameters.
Preservative
Effects of Curcumin on Semen of Hu Sheep
Animals | March 2024
Curcumin is a diketone compound found in
the rhizomes of ginger plants that can neutralize ROS and
improve the activity of endogenous antioxidant enzymes in the
body. Curcumin can also promote Nrf2 nuclear transport by
inhibiting the binding of Keap1 to Nrf2, thereby upregulating
the Nrf2/ARE pathway, which increases the levels of HO-1 and
NQO1 and improves antioxidant activity in the body. Curcumin has
been widely used in feed additives, which can enhance the
antioxidant capacity of animals and promote the nutrient
absorption rate in the gut. These results show that adding an
appropriate concentration (20 µmol/L) of curcumin to sheep semen
can affect sphingosine-1-phosphate, dehydroepiandrosterone
sulfate, phytosphingosine, and other metabolites of semen,
inhibit ROS production, and prolong the time of
cryopreservation.
Cardioprotective Effects of Curcumin Against
Diabetic Cardiomyopathies: A Systematic Review and Meta-Analysis
of Preclinical Studies
Traditional and
Western Medicine Center for Cardiovascular | March 2024
Meta-analysis showed that curcumin significantly improved
cardiac function indices, decreased markers of myocardial
injury, HW/BW ratio and randomized blood glucose compared to the
control group, in addition to showing beneficial effects on
mechanistic indices of myocardial oxidation, inflammation,
apoptosis, and autophagy. Curcumin may exert cardioprotective
effects in DCM through its antioxidant, anti-inflammatory,
autophagy-enhancing, and anti-apoptotic effects. Modern studies
have shown that curcumin can attenuate damage in DCM by being
anti-inflammatory(Wei et al., 2023), anti-oxidative stress(Wu et
al., 2022), anti-apoptosis(Ren et al., 2020), modulation of
autophagy(Sadeghi et al., 2023), and anti-fibrotic(Wang et al.,
2022). thus, curcumin emerges as a potential cardioprotective
candidate for ameliorating DCM. Our results suggest that
curcumin may play a cardioprotective 486 role in DCM through its
antioxidant, anti-inflammatory, autophagy-enhancing, and
anti-apoptotic effects.
Curcumin inhibits prostate cancer by upregulating
miR-483-3p and inhibiting UBE2C
Journal of
Biochemical and Molecular Toxicology | March 2024
Evidence
has shown the efficacy of curcumin in inhibiting the progression
of prostate cancer. In this research, curcumin was found
to suppress the proliferation and enhance the apoptotic rate in
in vitro prostate cancer cell models in a dose- and
time-dependent manner. In humans, the expression levels of
UBE2C are significantly higher in prostate cancer versus
benign prostatic hyperplasia. Treatment with curcumin decreased
the expression of UBE2C, whereas it increased miR-483-3p
expression. In summary, curcumin exerts its antitumor effects
through regulation of the miR-483-3p/UBE2C axis by decreasing
UBE2C and increasing miR-483-3p. The findings may also provide
new molecular markers for prostate cancer diagnosis and
treatment.
Curcumin and turmeric extract inhibit SARS-CoV-2
pseudovirus cell entry and spike-mediated cell fusion
bioRxiv | March 2024
Our study shows that turmeric extract
and curcumin are potential inhibitors of SARS-CoV-2 infection at
entry points, either by direct or indirect infection models.
Curcumin has been tested for its anti-SARS-CoV-2 activities by
plaque assay in Vero cells. Using the original virus, curcumin
inhibits SARS-CoV-2 infection. Curcumin may inhibit SARS-CoV-2
viral replication as indicated by reduced N protein expression
following viral infection. Here, we showed that curcumin and TE
reduced PSV entry in331 293T/hACE2/TMPRSS2 cells, in which 10 μM
curcumin and 10 μg/ml TE significantly affected the number of
GFP dots. From the previous studies, Marin-Palma et al. reported
that 10 μM curcumin can inhibit SARS-CoV-2 infection in Vero E6
cells. It is also reported that curcumin inhibits SARS-CoV-2
infection at concentrations of 3-10 μM.28. Furthermore, curcumin
inhibited PSV339 entry in 293/hACE2 cells. These results
corroborate curcumin effects against SARS-CoV-2340 infection
with our data representing curcumin inhibition at PSV cell entry
point. It has been known that curcumin affects the early stages
of viral replication cycles, including viral-receptor
attachment, internalization, and fusion that have been studied
against several types of viruses which involve influenza,
dengue, zika, chikungunya, pseudorabies, and VSV. Moreover,
curcumin and TE inhibit secondary infection via cell-to-cell
transmission in a syncytia formation model mediated by
SARS-CoV-2 spike expression. Cells treated with curcumin and TE
showed smaller syncytia with fewer nuclei than control cells.
Curcumin can also interact with SARS-CoV-2 spike. These data
align with our results that curcumin inhibited PSV350 entry and
syncytia formation. Our in vitro study using PSV and syncytia
models revealed that both curcumin and TE are potential
inhibitors of SARS-CoV-2 infection. Curcumin can interfere with
the spike-receptor binding during direct viral or
intercellular transmission, hindering viral infection and cell
fusion.
Interleukin-4 from curcumin-activated OECs emerges
as a central modulator for increasing M2 polarization of
microglia/macrophage in OEC anti-inflammatory activity for
functional repair of spinal cord injury
Cell Communication and Signaling | March 2024
Curcumin, a bioactive polyphenol extracted from rhizome of the
Curcuma longa, possesses a variety of pharmacological and
biological effects properties, such as anti-inflammatory,
antioxidant, anticancer, immunomodulatory, autophagy-enhancing,
and anti-microbial, etc. [34,35,36,37,38]. In addition to the
reported benefits, numerous studies have shown that Curcumin
exerts distinct neuroprotective and neutrophic effects on
neuronal cells and glia by modulating their related signalling
pathways [39,40,41]. Noteworthy, several recent reports have
demonstrated that Curcumin can improve OEC proliferation,
migration, morphologic changes, secretion of neurotrophic
factors and phagocytic activity [29, 42]. That is, Curcumin
significantly enhance the activation of OECs. In this regard,
Curcumin potentiates the beneficial behavior of OECs including
anti-inflammation and immunomodulation. As a result, aOECs could
function as the most promising candidates for cell-based
transplantation therapy targeting the CNS injury and
neurodegenerative diseases.
Investigation of the role of curcumin against the
toxicity induced by bisphenol a on the reproductive system in
male rats
Journal of Kerbala for
Agricultural Sciences | March 2024
The effect of curcumin as
a protective role against BPA on the oxidative stress parameters
showed that curcumin could decrease MDA concentration and at the
same time increase the concentration of both catalase and SOD.
Also, the results revealed that curcumin could increase the
concentration of LH, FSH, and testosterone as compared to the
BPA-treated group. Studies have demonstrated that curcumin
effectively addresses several male reproductive problems, hence
enhancing fertility. The positive benefits of curcumin may be
attributed to its capacity to scavenge free radicals and
function as an antioxidant agent, as well as its ability to
elevate serum testosterone levels. Curcumin mitigated the impact
of BPA and suppressed the antioxidant enzyme system in the host
tissues. This resulted in a noteworthy decrease in MDA levels
and a significant increase in catalase and SOD levels compared
to the rats treated with BPA. Therefore, curcumin partially
prevented the adverse effects caused by BPA. The presence of
phenolic compounds in curcumin's structures is a crucial element
in its ability to scavenge oxygen-derived free radicals that
cause cell lipid peroxidation. Curcumin exhibits a protective
impact on the enzymes SOD and CAT, which are reduced as a result
of BPA induction. Additionally, curcumin decreases lipid
peroxidation, specifically MDA. When curcumin is used together
with other drugs, there is a considerable increase in
antioxidant enzyme levels and a reduction in oxidative stress.
Using Curcumin as an antioxidant to counteract the effects of
BPA has a positive impact by effectively neutralizing the
harmful free radicals and maintaining the sexual hormones at
their optimal levels.
Efficacy and safety of curcumin therapy for knee
osteoarthritis: A Bayesian network meta-analysis
Journal of Ethnopharmacology | March 2024
Compared with
placebo, curcumin significantly reduced the visual analogue
scale pain score and total WOMAC score. Compared with NSAIDs,
curcumin and curcumin + NSAIDs had a reduced incidence of
adverse reactions. The surface under the cumulative ranking
curve results indicated that curcumin monotherapy, curcumin +
chondroprotective agents, and curcumin + NSAIDs have good
clinical value in osteoarthritis treatment. Curcumin, either
alone or in combination with other treatments, is considered to
have good clinical efficacy and safety in osteoarthritis
treatment. Drug combinations containing curcumin may have the
dual effect of enhancing efficacy and reducing adverse
reactions. Curcumin has anti-inflammatory, antitumour,
antioxidant, lipid-regulating, anticoagulant and other
pharmacological effects (Benameur et al., 2023; Luo et al.,
2023; Moballegh Nasery et al., 2020; Zhang et al., 2018).
Curcumin is a bioactive natural substance with great potential
clinical application value and is widely used in traditional
Chinese medicine to treat osteoarthritis and rheumatoid
arthritis (Akaberi et al., 2021; Feng et al., 2022; Henrotin et
al., 2022). Curcumin has anti-inflammatory and analgesic effects
similar to those of NSAIDs for arthritis and has a lower
incidence of adverse reactions (Chopra et al., 2013). Curcumin
is believed to exert an anti-inflammatory effect by inhibiting
the biological activity and synthesis of important proteases
that mediate the inflammatory process, such as cyclooxygenase-2,
lipoxygenase and inducible nitric oxide synthase (iNOS) (Paulino
et al., 2016). In addition, curcumin is believed to protect
cartilage (Comblain et al., 2016; Liu et al., 2018). Prior to
the current work, some clinical studies preliminarily confirmed
the clinical efficacy of curcumin in the treatment of arthritis
(Atabaki et al., 2020; Jamali et al., 2020). There are also
traditional head-to-head meta-analyses in the literature that
provide further evidentiary support for treating arthritis with
curcumin (Feng et al., 2022; Wang et al., 2021). In terms of
reducing RM use, curcumin, curcumin + NSAIDs and NSAIDs are
better than placebo, and curcumin + NSAIDs are better than
NSAIDs alone. These results indicate that the use of curcumin
may reduce the total amount of analgesic drugs used by knee
osteoarthritis patients. In terms of the incidence of adverse
reactions, both curcumin and curcumin + NSAIDs were lower than
NSAIDs alone. The results found that curcumin, curcumin + CP,
and curcumin + NSAIDs have good clinical value in treating knee
osteoarthritis. The results of SUCRA indicate that the two best
treatment measures for reducing VAS pain scores are curcumin and
curcumin + CP. Curcumin and NSAIDs are the preferred options for
reducing the total WOMAC score. Curcumin + NSAIDs is the best
medication for reducing the use of RM. Curcumin + NSAIDs and
curcumin + CP are the best two drug regimens for reducing the
incidence of AE. The above results suggest that curcumin can not
only have better clinical efficacy in the treatment of knee
osteoarthritis but also reduce the incidence of AE by its
combination with NSAIDs, but this situation still needs more
clinical data for verification. The conclusion of this NMA will
provide a new perspective for clinical decision-making on the
use of curcumin in the treatment of KOA, and we believe that the
combination of curcumin with other drugs should be studied
further. Experimental research (Guan et al., 2022; Hamdalla et
al., 2022; Zhang and Zeng, 2019) suggests that curcumin has a
good effect in the treatment of knee osteoarthritis, which can
promote chondrocyte proliferation, inhibit chondrocyte
apoptosis, inhibit the destruction of inflammatory factors on
cartilage, and maintain the balance of the internal environment
of articular cartilage. Shakibaei et al. found that curcumin can
inhibit the expression of MMP-3 and MMP-9 induced by IL-1β/TNF-α
in human chondrocytes (Shakibaei et al., 2007), which indicates
that curcumin can inhibit the decomposition of cartilage matrix
and play a role in treating KOA. The experiment with human
chondrocytes as the research object shows that curcumin can
downregulate the expression of inflammatory factors in cells,
such as nitric oxide, prostaglandin 2, TNF-a, IL-6, and IL-8,
reduce cartilage inflammation, and then inhibit cartilage damage
(Crivelli et al., 2019; Kim and Kim, 2019). In this context,
combined with our NMA results, we believe that curcumin has
great clinical application value in the treatment of knee
osteoarthritis and may become a substitute for NSAIDs.
Therapeutic advantages of curcumin, a polyphenol,
against traumatic brain injury through interaction with
different inflammatory signaling pathways and their effects on
levels of cytokines and related biomarkers
Molecular
Neurobiology | March 2024
The recent findings on curcumin
demonstrate its remarkable versatility as a molecule that
interacts with a variety of molecular targets. Various central
nervous system disease models have been shown to be susceptible
to curcumin's anti-inflammatory properties, including
intracerebral hemorrhage, global brain ischemia, and
neurodegeneration, all of which are associated with the
inflammation of the central nervous system. Similarly, curcumin
exerts neuroprotective effects in mammals when it crosses the
blood–brain barrier. Curcumin has been shown to reduce cerebral
edema, enhance membrane and energy homeostasis, and influence
synaptic plasticity following traumatic brain injury in studies.
Curcumin has been shown by numerous studies to be effective in
reducing inflammation. Research has shown that curcumin
suppresses the activation of NF-κB by inhibiting the
phosphorylation and degradation of IκB; as a result, curcumin
reduces the inflammation caused by NF-κB. The effects of
curcumin have been shown to be not only reduced post-traumatic
brain injury neuroinflammation, but also decreased levels of
inflammatory mediators that are produced following a traumatic
brain injury. The anti-inflammatory effects of curcumin were
demonstrated in an in vitro study using 100 mg/kg of curcumin.
According to the report, curcumin was able to reduce the amounts
of damage after traumatic brain injury induction and apoptosis,
particularly in the cortical cell model derived from embryonic
15-day pregnant mice, resulting in reduced damage. One study in
rats with traumatic brain injury administered 30 and 50 mg/kg of
curcumin daily for 35 days reduced levels of NLRP3, IL-1β, IL-6,
IL-18, and TNF-α. There was a reduction in neuroinflammation and
subsequent complications of TBI when curcumin was administered.
Furthermore, curcumin (at 500 mg/kg/day) decreased injury in the
ipsilateral cortex as a result of its ability to enhance
neutrophil infiltration (weight drop model-TBI). Curcumin
reduced apoptosis in cells and increased antioxidant activity,
suggesting potential neuroprotective effects. The pain levels
decreased when curcumin (50 mg/kg) was administered to
surgically induced traumatic brain injury rats. Curcumin has
been found to inhibit non-selective histone acetyltransferases,
suggesting potential benefits through histone acetylation. In a
rat model of TBI induced by FPI, curcumin (100 mg/kg) exerted
neuroprotective properties by activating the Nrf2 pathway. BDNF,
synapsin I, and CREB levels were reduced in animal models
following TBI after curcumin treatment (500 ppm). When given
oral doses of 100 mg/kg of curcumin per day, oxidative damage
was decreased, and omega-3 fatty acid DHA levels and 4-HNE (an
indicator of membrane lipid peroxidation) increased. Curcumin
supplements may have neuroprotective effects after brain injury
and may increase the activity of docosahexaenoic acid and fatty
acid-transport protein. According to Sharma et al.'s study
published in 2010, curcumin reduced iPLA2, 4-HNE, and STX-3,
which improved learning progress and relieved complications
associated with traumatic brain injury. Several studies, such as
the one by Zahedi et al., suggest promising effects of curcumin,
developing effective therapeutic strategies necessitates
rigorous preclinical studies and clinical trials.
Curcumin mitigates acrylamide-induced ovarian
antioxidant disruption and apoptosis in female Balb/c mice: A
comprehensive study on gene and protein expressions
Food Science & Nutrition | March 2024
Curcumin is known for
its antioxidant properties.Evidence has shown that curcumin
exhibits anti-inflammatory, antioxidants, anti-cancer activities
(Khajeh pour et al., 2023; Mailafiya et al., 2023). These
advantageous features are attributed to its elevated antioxidant
activity, stemming from the hydroxyl and methylene groups within
the β-diketone moiety present in its chemical structure (Chen et
al., 2023; Wang et al., 2017). There are several studies that
explored the positive effects of curcumin on the ovary tissue.
Azami et al. (2020) showed that curcumin could prevent ovarian
aging by increasing ovarian volume and the number of follicles.
Lv et al. (2021) also suggested that curcumin had beneficial
effects on the ovary and reproductive organs by regulating the
PTEN-AKT-FOXO3a pathway. Furthermore, ACR exposure led to a
significant increase in estradiol, luteinizing hormone,
testosterone, ovarian tumor markers such as CA125, and
carcinoembryonic antigen (CEA), while serum progesterone,
follicle-stimulating hormone, and total antioxidant capacity
decreased in female rats. Curcumin treatment restored
serological indices toward normal levels (Elsawi et al., 2023).
In our study, ACR exposure adversely affected ovarian
antioxidant defense thereby leading to increased pro-apoptotic
markers. Notably, curcumin treatment effectively mitigated these
effects, restored antioxidant potential, and reduced
acrylamide-induced toxicity in female mouse ovaries. Our
findings indicate that the administration of curcumin at doses
of 100 and 200 mg/kg efficiently restores the expression of
antioxidant genes, demonstrating a significant improvement at
the 200 mg/kg dose compared to the 100 mg/kg dose. Several
studies have illustrated the efficacy of curcumin in mitigating
oxidative stress-related concerns, encompassing factors like
total antioxidant capacity, malondialdehyde (MDA), and
superoxide dismutase (SOD) within physiological conditions (Chen
et al., 2022; Khayatan et al., 2023). In a recent systematic
review and meta-analysis of randomized controlled trials,
curcumin exhibited a substantial influence on indicators of
oxidative stress, including total antioxidant capacity,
malondialdehyde, and SOD levels (Dehzad et al., 2023). In
various animal models of ovarian diseases, curcumin has
demonstrated the ability to enhance overall ovarian function (Eser
et al., 2015; Wang et al., 2017). Furthermore, the
administration of curcumin has been proven beneficial in
addressing gynecological diseases in women, as highlighted in
studies (Kamal et al., 2021; Shen et al., 2022). For instance,
curcumin supplementation has beneficial effects on weight loss,
glucose and lipid metabolism, metabolic parameters, and androgen
levels in polycystic ovary syndrome patients (Ghanbarzadeh-Ghashti
et al., 2023; Sohaei et al., 2019). Curcumin causes a reduction
in serum nitric oxide release in women with primary dysmenorrhea
and premenstrual syndrome pain (Farrokhfall et al., 2023). It
also decreases the total score of primary symptoms of menopause
by affecting oxidative Stress Biomarkers (Farshbaf-Khalili et
al., 2022). Importantly, curcumin treatment emerged as a
promising therapeutic intervention, effectively restoring
antioxidant potential and mitigating acrylamide-induced toxicity
in the ovaries of female mice. These results contribute to a
deeper understanding of the molecular mechanisms involved in
ACR-induced reproductive toxicity and underscore the potential
of curcumin as a protective agent against such detrimental
effects.
Curcumin abrogates cobalt-induced neuroinflammation
by suppressing proinflammatory cytokines release, inhibiting
microgliosis and modulation of ERK/MAPK signaling pathway.
Journal of Chemical Neuroanatomy | March 2024
Curcumin
treatment significantly reduced tissue levels of proinflammatory
cytokines TNF-α and IL-1β. Microglial activation and ERK protein
upregulation induced by cobalt were attenuated by curcumin.
Results showed that curcumin abrogated neuroinflammation by
suppressing the release of proinflammatory biomarkers, reducing
microgliosis, and modulating the ERK MAPK pathway. Extensive
studies have suggested that curcumin possesses anti-inflammatory
(Wang et al., 2017), neuroprotective (Teter et al., 2019),
antioxidant (Memarzia et al., 2021), anti-cancer (Dou et al.,
2017), hepatoprotective (Macías-Pérez et al., 2019). Curcumin
significantly suppressed the release of neuroinflammation
markers and reduced microgliosis in the hippocampus of the rats.
In addition, the significant correlation between ERK expression
and proinflammatory biomarkers and microgliosis has offered some
mechanistic insight into curcumin's neuroprotective properties.
Curcumin in Cancer and Inflammation: An In-Depth Exploration of
Molecular Interactions, Therapeutic Potentials, and the Role in
Disease Management
International Journal of
Molecular Sciences | March 2024
Curcumin, a polyphenolic
compound, is extracted from the turmeric plant. The significance
of curcumin’s structure, especially its conjugated enol-ketone
system, lies in its ability to engage in diverse molecular
interactions. In colorectal cancer, early studies indicated
potential benefits of curcumin in reducing polyp number and size
in familial adenomatous polyposis (FAP) patients, suggesting its
role in managing precancerous lesions. For head and neck cancer,
particularly oral leukoplakia, curcumin showed a significant
clinical response compared to placebo. It is also noteworthy
that the combination of clinical and histological responses
indicated a more pronounced effect of curcumin, suggesting its
potential utility in the early-stage management of oral
leukoplakia. In the context of multiple myeloma, particularly in
patients with monoclonal gammopathy of undetermined significance
(MGUS) or smoldering multiple myeloma (SMM), curcumin
demonstrated some promise. The promising results in certain
areas warrant continued research to optimize curcumin’s
formulation, delivery, and dosing to maximize its clinical
benefits.
Curcumin effects on glycemic indices, lipid profile,
blood pressure, inflammatory markers and anthropometric
measurements of non-alcoholic fatty liver disease patients: A
systematic review and meta-analysis of randomized clinical trial
Complementary Therapies in Medicine Volume | March 2024
Curcumin supplementation was associated with significant changes
in fasting blood glucose and homeostatic model assessment for
insulin resistance in adults with NAFLD. Curcumin
supplementation was associated with significant changes in
triglyceride, total cholesterol, and low density lipoprotein in
adults with NAFLD. Curcumin supplementation in doses of
50−3000 mg/day over 8–12 weeks was associated with significant
reductions in levels of FBG, HOMA-IR, TG, TC, LDL, weight and
BMI in patients with NAFLD. Previous studies have reported
curcumin as a safe complementary therapy for several diseases. A
systematic review suggested that curcumin is effective in
lowering low density lipoprotein, cholesterol (LDL-C), TG,
fasting blood glucose (FBG), homeostatic model assessment for
insulin resistance (HOMA-IR), and weight in NAFLD patients, and
it is well tolerated. Another study revealed that curcumin
supplementation has favorable effects on metabolic markers and
anthropometric parameters in patients with NAFLD. Also,
results of a randomized controlled trial suggest that curcumin
supplementation reduces serum lipids in patients with NAFLD.
A study showed curcumin supplementation has a favourable effect
on total cholesterol (TC), and BMI in participants with NAFLD.
Therefore, promoting curcumin as adjuvant treatment on NAFLD
patients might be justified. In conclusion, our study indicated
that curcumin supplementation in doses of 50 − 3000 mg/day over
8–12 weeks was associated with significant changes in FBG,
HOMA-IR, TG, TC, LDL, weight and BMI in adults with NAFLD.
Curcumin suppresses metastasis of triple-negative
breast cancer cells by modulating EMT signaling pathways: An
integrated study of bioinformatics analysis
Medicine | February 2024
Curcumin demonstrates potential
anticancer properties in the treatment of triple-negative breast
cancer cells. The findings suggest that the inhibitory effect of
curcumin on the motility of triple-negative breast cancer cells
could be attributed to the concurrent downregulation of specific
signaling pathways, through influencing the EMT signaling
process. This study employs a comprehensive approach that
integrates bioinformatics analysis with in vitro experimental
methodologies, providing substantial evidence supporting
curcumin’s potential in breast cancer therapy. Curcumin exhibits
its therapeutic potential in triple-negative breast cancer cells
by modulating multiple signaling pathways. These findings
provide experimental evidence for considering curcumin as a
potential therapeutic strategy in the treatment of
triple-negative breast cancer cells. This study concentrates on
curcumin, a yellow phenolic pigment derived from turmeric,
renowned for its anti-inflammatory, antioxidant, and
immunomodulatory properties. Recent research has shown that
curcumin is crucial in treating several tumors by modulating
typical cell biological effects such as cell proliferation,
apoptosis, cell cycle, and metastasis. Thus, curcumin may play a
significant role in the initiation and progression of various
cancers, including breast, lung, and liver cancers, through
affecting multiple signaling and molecular pathways, such as Rb,
P53, mitogen-activated protein kinase, phosphatidylinositol 3-K
(PI3K)/protein kinase B, and NF-kappaB (nuclear factor kappa B
cells, NF-κB). Previous studies have demonstrated curcumin’s
capacity to inhibit cell proliferation and invasion in human
triple-negative breast cancer cells. This study is significant
for its exploration of curcumin’s potential clinical
applications in treating triple-negative breast cancer cells. By
elucidating the antitumor mechanisms of curcumin in TNBC cells,
it establishes a crucial foundation for developing innovative
therapeutic strategies. Curcumin significantly inhibited the
proliferation of Hs578T and MDA-MB-231 cells. Flow cytometry
results showed that curcumin induced apoptosis in these cells
and arrested the cell cycle at the G2/M phase. Additionally,
Transwell assay results showed that curcumin effectively reduced
the motility of Hs578T and MDA-MB-231 cells. Enrichment analysis
of RNA sequencing data showed that the mechanism of action of
curcumin was significantly associated with signaling pathways
such as pathways in cancer, focal adhesion, and PI3K-Akt
signaling pathways. Finally, Western blotting analysis showed
that curcumin significantly decreased the expression levels of
key proteins including Fibronectin, mTOR, β-Catenin, p-Akt, Akt,
N-Cadherin, p-S6, and S6. Curcumin, an efficacious
anticancer compound isolated from turmeric rhizomes, mediates
its therapeutic effects through the modulation of various
cellular pathways. These include the suppression of tumor
metastasis, angiogenesis, and inflammation, alongside the
modulation of apoptosis, cell cycle progression, and resistance
to multiple drugs. The role of curcumin as a cancer
chemopreventive agent has been rigorously studied in various
cancer models. Recent studies have highlighted curcumin’s
antiproliferative and antimotility effects on breast cancer
cells. In this study, our objective is to elucidate the
antitumor properties of curcumin, focusing on its potential
mechanisms of action and therapeutic efficacy. Employing gene
enrichment analysis, incorporating both Gene Ontology and Kyoto
Encyclopedia of Genes and Genomes pathways, on RNA sequencing
data, we determined that curcumin predominantly acts through
pathways such as Pathways in cancer, Focal adhesion, PI3K-Akt
signaling pathway, and cell cycle. he CCK8 assay indicated that
curcumin markedly reduces the cellular activity of TNBC cells.
In parallel, Western Blot analysis revealed that curcumin lowers
the expression of key proteins, including N-Cadherin,
Fibronectin, β-Catenin, p-Akt, Akt, mTOR, p-S6, and S6, in
Hs578T and MDA-MB-231 cells. These proteins play crucial roles
in the EMT process and its related signaling pathways,
particularly the PI3K/Akt/mTOR pathway. The results imply that
curcumin’s inhibitory effect on the motility of triple-negative
breast cancer cells may stem from the simultaneous
downregulation of these signaling pathways, impacting the EMT
signaling cascade.
Curcumin Enhances the Anti-Cancer Efficacy of CDK4/6
Inhibitors in Prostate Cancer
Archivos
Españoles de Urología | February 2024
This study aimed to
investigate the potential of combining cyclin-dependent kinase 4
and 6 (CDK4/6) inhibitors with curcumin, a natural compound
known for its anti-aging properties, to enhance the anti-cancer
efficacy in prostate cancer. Curcumin is a natural compound
extracted from turmeric, which has been shown to have multiple
biological activities, including anti-inflammatory, antioxidant
and anti-cancer effects. The synergistic effect observed in this
study suggested that curcumin may enhance the therapeutic
efficacy of CDK4/6 inhibitors in prostate cancer treatment,
further proving the previously reported findings. Furthermore,
this study revealed that curcumin regulated cellular aging
induced by CDK4/6 inhibitors through the inhibition of the mTOR
and STAT3 pathways, which was also reported in previous studies.
Similar conclusions were drawn in this study, which showed that
curcumin could modulate cellular senescence. Interestingly,
curcumin contributed to the anti-cancer effects of CDK4/6
inhibitors in prostate cancer treatment. Moreover, this study
revealed that curcumin could inhibit the stemness
characteristics of cancer cells induced by LY CM, as evidenced
by the downregulation of cancer stem cell markers ALDH1A1, CD44
and Nanog. The ability of curcumin to target cancer stem cells
provides further evidence of its potential as an effective
therapeutic agent in prostate cancer treatment. As previously
reported, the combination of curcumin and LY CM can inhibit the
dryness of cancer cells. Overall, the findings of this study
support the notion that combining CDK4/6 inhibitors with
curcumin may have clinical implications for the treatment of
prostate cancer. The findings of this study provide valuable
insights into the potential of combining CDK4/6 inhibitors with
curcumin in the treatment of prostate cancer.
The complex effect of polyphenols on the gut microbiota and
triggers of neurodegeneration in Parkinson’s disease
Zhurnal Nevrologii | February 2024
A promising
direction for influencing microflora and inflammatory changes in
the intestine is the use of polyphenols, primarily curcumin. The
review of experimental, laboratory, clinical research proving
the pleiotropic effect of curcumin, including its antioxidant,
anti-inflammatory, neuroprotective effects, realized both
through peripheral and central mechanisms is presented.
Immunomodulatory effects of curcumin on macrophage
polarization in rheumatoid arthritis
Frontiers in Pharmacology | February 2024
As a natural
compound, curcumin is favorable for improving symptoms in
rheumatoid arthritis patients due to its potent efficacy,
affordability, and minimal side effects. In addition, curcumin
has been reported to ameliorate several diseases via epigenetic
regulation, encompassing: (1) suppression of DNMTs; (2)
regulation of histone acetyltransferases and histone
deacetylases; and (3) regulation of miRNAs (Boyanapalli and
Kong, 2015). Several studies have shown that curcumin can be
used in cancer treatment to reverse DNA methylation, alter
histone modifications and target miRNA expression (Shu et al.,
2011; Bao et al., 2012; Yu et al., 2013). Curcumin has been
extensively demonstrated to possess anti-inflammatory,
antioxidant, immunomodulatory and anticancer properties in both
experimental and clinical studies (Xu et al., 2018). Curcumin
has shown strong therapeutic potential, especially in autoimmune
diseases, such as RA and systemic lupus erythematosus (SLE)
(Yang et al., 2019; Chamani et al., 2022; Kou et al., 2023). A
large number of investigations have indicated that curcumin
modulates macrophage polarization and function to alleviate
inflammation and therefore can be used to treat
inflammation-related diseases (Gao et al., 2015; Karuppagounder
et al., 2016; Abdollahi et al., 2023). Curcumin regulates the
functions of various immune cells, including macrophages
(Mohammadi et al., 2019), dendritic cells (DCs) (Rahimi et al.,
2021), B cells (Mohammadi et al., 2022) and T cells (Rahimi et
al., 2019), thereby modulating both innate and adaptive immunity
(Shehzad and Lee, 2013). The anti-inflammatory activity of
curcumin is due to its suppression of multiple signalling
molecules, including NF-κB, activated protein (AP)-1, MAPKs, and
protein kinase C (Kahkhaie et al., 2019). In addition, curcumin
is a potent inhibitor of reactive-oxygen-generating enzymes,
such as lipoxygenase, cyclooxygenase (COX), xanthine
dehydrogenase and iNOS (Rao, 2007). As shown in macrophages,
curcumin inhibits LPS and IFN-γ-induced nitric oxide production.
Curcumin mediated dendritic cell maturation by
modulating cancer associated fibroblasts
Journal of Cancer Research and Therapeutics | February 2024
Curcumin, traditionally known for its anti-inflammatory effects,
has been shown to be a potent immunomodulatory agent that can
modulate the activation of various immune cells by
downregulating pro-inflammatory cytokines, including IL-1, IL-2,
IL-6, IL-8, IL-12. In our previous studies, we have reported on
curcumin's different potential to target cancerous cells in
Acute myeloid leukemic (AML) patients as well as lung cancer
patients. Moreover, several reports have suggested that
curcumin-treated DCs produce significantly lower levels of IL-6,
IL-12, IL-10, and TNFα, thus creating a Th2 permissive
environment. In addition, there are several other reports
confirmed that curcumin inhibited IL-1b, IL-6 through modulation
of NF-kB and MAPK pathways. Moreover, curcumin-inhibited
IL-6-induced STAT3 phosphorylation and nuclear translocation in
multiple myeloma cells. Recently, Paul S and Sa G revealed that
curcumin acting as an adjuvant in immunotherapies may present
with novel approaches for researchers and clinicians to obtain
improved treatment outcomes. Collectively, our results concur
with previous findings reported by other researchers, signifying
curcumin’s potential to decrease the secretion of IL-6, IL-10,
and TGF-β in conditioned media, this being mediated via
curcumin’s efficacy to instruct cancer associated fibroblasts to
secrete exosomes resulting in the creation of an
immunomodulating TME from an immunosuppressive TME environment.
This study may provide basis to revise fundamental treatment
rationales and formulate synergistic therapeutic approaches by
using curcumin along with DC-based immunotherapies to overcome
cellular resistance in cancer treatment but it still requires
more in-depth research to have an in-sight into mechanism, which
might open up a newer avenues for curcumin’s use as an
appropriate immunotherapeutic modulator.
Exploring the therapeutic potential of curcumin in
oral squamous cell carcinoma
Pathology -
Research and Practice | February 2024
Curcumin, a
polyphenolic compound derived from rhizomes of Curcuma longa
(turmeric), has garnered significant attention due to its
potential as an anticancer agent. Its pleiotropic properties,
including anti-inflammatory, antioxidant, and anti-tumor
activity, make it a compelling candidate for exploring novel
strategies in the oral cancer treatment. Of particular interest
is curcumin’s capacity to interfere with the intricate web of
cellular signaling pathways involved in angiogenesis and tumor
progression. Our study highlights the intriguing possibility of
curcumin being a multifaceted medicinal supplement in the fight
against oral cancer. Through its modulation of HIF-1α, VEFG,
STAT3, and MMP-3, curcumin shows promise in disrupting critical
pathways associated with angiogenesis and tumor progression,
offering new avenues for targeted cancer treatments. These
findings underscore curcumin’s significance in oral cancer
research and its potential for future clinical applications.
Curcumin
in Alzheimer’s Disease and Depression: Therapeutic Potential and
Mechanisms of Action
Brazilian Archives of
Biology and Technology | February 2024
Curcumin is a
polyphenol present in Curcuma longa, a root used in Asian
cuisine for thousands of years, and it has several medicinal
properties, acting as an antioxidant, anti-inflammatory,
anticancer, among others. The aim of this study was to evaluate
the effects of curcumin in Alzheimer's disease and Depression,
which has as its main pathogenesis the reduction of BDNF levels,
monoamine levels, increased oxidative stress, inflammation,
beta-amyloid aggregation, Tau protein accumulation and aluminum
neurotoxicity, verifying its therapeutic capacity. Therefore, a
literature review was performed in the Scholar Google,
ScienceDirect, and PubMed databases. The data analyzed
demonstrated that curcumin supplementation is able to restore
BDNF levels in Alzheimer's disease and depression, in addition
to modulating monoamines and reducing oxidative stress,
inflammation, beta-amyloid aggregation, Tau protein accumulation
and aluminum neurotoxicity, improving their symptoms.
The Protective Effects of Curcumin against Renal Toxicity
Current Medicinal Chemistry | February 2024
Curcumin is a
naturally polyphenolic compound used for hepatoprotective,
thrombosuppressive, neuroprotective, cardioprotective,
antineoplastic, antiproliferative, hypoglycemic, and
antiarthritic effects. Kidney disease is a major public health
problem associated with severe clinical complications worldwide.
The protective effects of curcumin against nephrotoxicity have
been evaluated in several experimental models. In this review,
we discussed how curcumin exerts its protective effect against
renal toxicity and also illustrated the mechanisms of action
such as anti-inflammatory, antioxidant, regulating cell death,
and anti-fibrotic. This provides new perspectives and directions
for the clinical guidance and molecular mechanisms for the
treatment of renal diseases by curcumin.
Effect of Curcumin on the Process of
Neuroinflammation Caused by COVID-19
Curcumin and Neurodegenerative Diseases | February 2024
Curcumin, the main active ingredient of Curcuma longa L., has
antioxidant, anti-inflammatory, antitumor, antiviral,
neuroprotective, and immune system-modulating properties.
Because of these properties, this curcuminoid may be helpful
both before and after SARS-CoV-2 infections, enhancing health
status in the various complications brought on by the illness
(respiratory, enteric, hepatic, and neurological). From this
perspective, this chapter aims to report on findings regarding
the effect of turmeric on COVID-19-induced neuroinflammation.
Some studies highlight that curcumin is effective in COVID-19
conditions by inhibiting inflammatory and neuroinflammatory
signaling pathways, such as nuclear factor kappa B (NF-kB), and
the induction of various pro-inflammatory cytokines and
chemokines, including interleukin (IL)-6, interferon (IFN) γ,
monocyte chemoattractant protein (MCP)-1, tumor necrosis factor
(TNF)-α and IL-1β.
Effect of Curcumin on Dysmenorrhea and Symptoms of
Premenstrual Syndrome: A Systematic Review and Meta-Analysis
Korean Journal of Family Medicine | February 2024
The
reduction in the severity of PMS and dysmenorrhea has been
attributed to curcumin’s anti-inflammatory and antidepressant
activities. Studies have found that curcumin can alleviate
symptoms of dysmenorrhea and PMS. Evidence shows that curcumin
has pleiotropic effects and acts as an anti-inflammatory,
anticancer, antioxidant, and antibacterial agent. In addition,
curcumin can reduce prostaglandin production. Khayat et al.found
that curcumin can alleviate symptoms experienced before
menstruation without any adverse effects. Furthermore, curcumin
in women with PMS may also regulate neurotransmitters and
biomolecules, exert antioxidant and analgesic effects, and
reduce oxidative stress. In conclusion, the findings of our
meta-analysis showed that using various forms of curcumin could
reduce the severity of PMS and dysmenorrhea owing to its
anti-inflammatory and antidepressant activities. The present
study revealed that curcumin consumption immediately before or
during menstruation could alleviate the severity of
dysmenorrhea. Therefore, it could be regarded as an appropriate
alternative for women with PMS and dysmenorrhea to improve their
quality of life.
Review of the Protective Mechanism of Curcumin on
Cardiovascular Disease
Drug Design,
Development and Therapy | February 2024
Curcumin has been
shown to have a variety of pharmacological properties over the
past decades. Curcumin can significantly protect cardiomyocyte
injury after ischemia and hypoxia, inhibit myocardial
hypertrophy and fibrosis, improve ventricular remodeling, reduce
drug-induced myocardial injury, improve diabetic
cardiomyopathy(DCM), alleviate vascular endothelial dysfunction,
inhibit foam cell formation, and reduce vascular smooth muscle
cells (VSMCs) proliferation. Clinical studies have shown that
curcumin has a protective effect on blood vessels. Toxicological
studies have shown that curcumin is safe. Curcumin is a
bioactive component of the curry spice, and its pleiotropic
effects in cardiovascular diseases suggest that it is a
promising drug candidate. Specifically, curcumin can
significantly alleviate vascular endothelial dysfunction,
inhibit foam cell formation, reduce VSMCs proliferation, protect
cardiomyocyte injury after ischemia and hypoxia, inhibit
myocardial hypertrophy and fibrosis, improve ventricular
remodeling, reduce drug-induced myocardial injury, and improve
DCM. The therapeutic effect and mechanism of curcumin have
become the focus of pharmacokinetic research. Curcumin has a
good therapeutic effect, especially in the cardiovascular field.
Previous reviews have summarized the protective effects of
curcumin on CVDs. In 2020, Li et al reviewed the preclinical
studies of curcumin in CVDs such as cardiac hypertrophy, heart
failure, abdominal aortic aneurysm, stroke, drug-induced
cardiotoxicity, and diabetic cardiovascular complications, and
reviewed the potential molecular targets of curcumin.
Pourbagher-Shahri et al also reviewed the effects of curcumin on
the cardiovascular system in 2021. The protective mechanism of
curcumin against cardiovascular diseases is complex and
networked. It is important to note that curcumin is a
complementary or alternative medicine, not a replacement for the
main treatment, and needs to be used under the guidance of a
doctor. To be sure, curcumin is an important drug that is worth
exploring.
Curcumin alleviates atrazine-induced cardiotoxicity
by inhibiting endoplasmic reticulum stress-mediated apoptosis in
mice through ATF6/Chop/Bcl-2 signaling pathway
Biomedicine & Pharmacotherapy | February 2024
Curcumin
displays promise in alleviating heart injury by alleviating
cardiac apoptosis. Curcumin, acclaimed for its pronounced
anti-inflammatory and antioxidant properties, has garnered
interest as a potential therapeutic agent. Collectively, our
findings illuminate curcumin’s cardioprotective effect against
ATR-induced injury, primarily through its anti-ERS and
anti-apoptotic activities, underscoring curcumin’s potential as
a therapeutic for ATR-induced cardiotoxicity. Curcumin has many
biological benefits, such as antioxidant, anti-inflammation and
immunoregulatory effect. Research has shown that curcumin can
help reduce damage to various systems in the body, such as the
cardiovascular, digestive, and respiratory systems. In addition,
adding the curcumin to the diet can significantly reduce the
body weight and body fat rate of mice which fed with high-energy
diet, and alleviate inflammation and diabetes in mice.
Meanwhile, curcumin significantly decreased the expression of
inflammatory factors induced by tumor necrosis factor-α in
adipocytes and the differentiation of preadipocytes. Mortezaee
and his colleague’s study shown that curcumin inhibits the
growth of tumor cells. Curcumin has various pharmacological
effects, including reducing inflammatory reactions and oxidative
stress, enhancing immunity, and combating infections. Recent
studies have shown that the cardioprotective benefits of
curcumin are facilitated through myriad mechanisms, among which
include the modification of gut microbiota makeup, inducement of
macrophage polarization, alleviation of oxidative stress, and
modulation of pivotal signaling pathways such as AMPK and mTOR.
In doing so, curcumin minimizes damage to the subcellular
structures within the myocardium. The present study revealed
that curcumin can alleviate the ATR-induced damage in cardiac
cells. This mitigation manifests as reductions in inflammatory
cell infiltration and subcellular membrane damage (including
mitochondria, endoplasmic reticulum, and nucleus) instigated by
ATR. Most notably, curcumin appears to have the capacity to
remediate myocardial damage.
Improving cognitive function with intermittent dose
escalation of curcumin extract in chemotherapy-induced cognitive
impairment patients: a randomized controlled trial
Advances in Traditional Medicine | February 2024
The group of
subjects receiving curcumin extract experienced clinically and
statistically significant improvements in cognitive function.
Administration of curcumin extract with intermittent dose
escalation regimen proved to be safe and able to improve
cognitive function of cognitive impairment patients clinically
and statistically significant. Recently, there has been multiple
pharmacological studies done to investigate the antioxidant,
antiinflamation, anticarcinogenic, and anti-bacterial effects of
curcumin. Curcumin is a safe natural product to be consumed by
humans (Abd El-Hack et al. 2021). Curcumin is also known to
increase the effectiveness of chemotherapeutic agents via
increasing cancer cells sensitization against chemotherapy and
protecting normal cells from chemotherapy damage (Tan and
Norhaizan 2019). Curcumin extract has several advantages,
including having a pleiotropic effect as anti-inflammatory,
antioxidant, and antiapoptotic (Panahi et al. 2021; Abd El-Hack
et al. 2021). Furthermore, curcumin extract also has
anti-carcinogenic effects. Thus, the combination of various
mechanisms of action makes curcumin extract a comprehensive
therapeutic modality to prevent or treat cellular damage,
especially due to exposure to neurotoxic and oxidative stress
chemotherapeutic agents (Liu et al. 2019; Akbari et al. 2020).
In addition, curcumin extract also has a low toxicity index, so
it has a broad and safe therapeutic window profile. Clinically,
curcumin extract has been widely tested and is known to have
neuroprotective effects. For example, curcumin extract can
inhibit cognitive function impairment caused by the oxidative
stress of cigarette smoke (Muthuraman et al. 2019). In the
context of chemotherapy, curcumin has been shown to improve
neurogenesis and synaptogenesis that play a role in brain
plasticity, as well as increase hippocampal autophagy so that it
can suppress the process of apoptosis in the central nervous
system, after exposure to cisplatin-based chemotherapy (Yi et
al. 2020). Thus, administration of curcumin extract in carcinoma
patients undergoing chemotherapy regimens can help prevent, even
improve, the symptoms of cognitive impairment. Based on the
results of the analysis of this study, valid evidence was found
that the administration of curcumin extract with the new regimen
was proven to be safe and effective in improving cognitive
function in patients with cognitive impairment due to
carboplatin-paclitaxel chemotherapy regimen.
Administration of curcumin extract with a dose escalation system
was proven to improve cognitive function of patients with
clinical and statistical significance. Administration of
curcumin extract with this dosage regimen has also been shown to
have a good safety profile.
A Review on Medicinal Benefits of Curcumin on Cancer
International Research Journal of Modernization in Engineering
Technology and Science | February 2024
Curcumin, a natural
polyphenol found in the food spice turmeric, has been shown to
inhibit the survival and proliferation of cancer cells and
trigger apoptosis without promoting side effects. In this
context, the regulation of the cell cycle and its modulation by
curcumin has attracted great attention in recent years. Curcumin
can inhibit cancer cells, cause apoptosis, inhibit angiogenesis,
inhibit the expression of anti-apoptotic proteins, as well as
inhibit the immune system of cancer cells. Various studies have
reported the antitumor activity of curcumin against breast
cancer, lung cancer, head and neck squamous cell carcinoma,
prostate cancer, and brain tumors. Curcumin and its derivatives
have attracted great attention in the last two decades due to
their biofunctional properties such as antibacterial, antifungal
and antiviral. Curcumin exerts its anti-cancer effect through
various mechanisms. Curcumin can inhibit the growth of many
cancer cells by reducing the regulation of anti-apoptotic cells,
activating caspases, and promoting tumor suppressors such as
P53. Curcumin has properties that scavenge free radicals and
therefore may play an important role in inhibiting the growth of
cancer. Many cellular and preclinical studies have shown that
curcumin can prevent DNA damage caused by oxidative factors
(such as ionizing radiation) by inhibiting free radicals and
reactive oxygen species. Curcumin prevents cancer formation by
inhibiting the formation of NF-kappaB. Curcumin prevents tumor
formation and growth by inhibiting and activating two enzymes
(Phase 1 and Phase 2). The motivation to use curcumin
stems not only from its therapeutic potential, but also from the
fact that curcumin is more easily absorbed by patients without
the side effects of many other medicinal products such as
nausea, vomiting, diarrhea, hair lossand more serious long-term
conditions such as liver failure. Various studies comparing
breast cancer incidence and cancer rates in India and the West
show that the risk of breast cancer is lower in India. Curcumin
can interact with various biochemical pathways of cancer cells
and survive by directly or indirectly binding to different
targets. Curcumin has been shown to interact with many targets,
including transcription factors, growth factors, DNA, RNA, and
many proteins involved in cell signaling pathways. The chemical
structure of curcumin has diverse properties that make it highly
effective and has many synergistic properties for various
molecular targets. Curcumin, the active ingredient of turmeric
extract, has been used for many years as anti-inflammatory,
antioxidant, anti-cancer and more. Although there is much to
learn about curcumin and its therapeutic properties, it has
great potential in the treatment and prevention of cancer.
Curcumin, a natural product, is not only non-toxic but also has
many effects on various pathways involved in tumorigenesis.
Curcumin Improves Chemotherapy Resistance in Breast
Cancer Cells via Inhibiting the Secretion of FGF2/FGFR2 from
Cancer-Associated Fibroblasts
Journal of
Biological Regulators and Homeostatic Agents | February 2024
In this experiment, the data also showed that curcumin
effectively blocked breast cancer cell proliferation, induced
apoptosis, and boosted the breast cancer cell sensitivity to
PTX. Curcumin increases breast cancer cell sensitivity to PTX by
inhibiting the secretion of FGF2/FGFR2 from CAFs. Curcumin is a
polyphenol isolated from turmeric that belongs to the rhizome of
Zingiberaceae. In vivo and in vitro
investigations have demonstrated a variety of pharmacological
activities of curcumin. In addition to low toxicity, curcumin
has pharmacological actions, including anti-inflammation,
antioxidation, anti-hyperlipidemia, anti-atherosclerosis,
anti-tumor, anti-HIV virus, and other beneficial properties.
Many scholars have suggested that curcumin can be utilized to
treat malignant tumors and chronic diseases. In vitro
and in vivo experiments verified that curcumin has a
clear anti-tumor activity. It was reported that curcumin
inhibits the development of breast cancer through modulation of
multiple molecular targets, including p53, Wnt/β-catenin, and
PI3K/Akt/mTOR signaling pathways. Curcumin exhibits diverse
pharmacological properties, including anti-inflammation,
antioxidant, and anti-tumor. Curcumin exhibits diverse
pharmacological properties, including anti-inflammation,
antioxidant, and anti-tumor. Zou et al. focused on the
effect of curcumin on the breast cancer cell sensitivity to
cisplatin and discovered that curcumin enhanced the cisplatin
sensitivity. In addition, curcumin reversed the enhanced
resistance in breast cancer cells induced by CAFs-CM. We further
detected the roles of FGF2 and its receptor FGFR2 in the
chemo-sensitization of curcumin. Accordingly, curcumin may
increase the breast cancer cell sensitivity to PTX by inhibiting
the secretion of FGF2 and FGFR2 by CAFs. It provides a new idea
for combining curcumin with chemotherapy. This study revealed
the potential of curcumin in cancer therapy and provided a
foundation for combining curcumin with chemotherapy in the
treatment of breast cancer.
The Combination of Vitamin D and Curcumin Piperine Attenuates
Disease Activity and Pro-inflammatory Cytokines Levels
Insystemic Lupus Erythematosus Patients
Current Rheumatology Reviews | February 2024
Curcumin-piperine might synergise with vitamin D to induce
clinical remission in patients with systemic lupus
erythematosus. Curcumin supplementation in premenopausal women
and dysmenorrhea improves vitamin D levels. Several studies have
reported the efficacy of vitamin D or curcumin for SLE
treatment. Curcumin is a phenolic compound widely found in
ginger, turmeric, and curcuma plants and has the potential as an
immunomodulator for the complementary treatment of SLE. Curcumin
acts as an activator or inhibitor of several transcription
factors that play a role in activating and differentiating Th1,
Th2, Th17, and Tregs. In a previous report, curcumin
synergistically interacts with vitamin D because it is also a
natural ligand for the vitamin D receptor (VDR). Previous
studies reveal that administering a combination of curcumin and
vitamin D3 resulted in a better recovery of neuronal cells from
Alzheimer's disease. Another study demonstrates that curcumin
supplementation in premenopausal women and dysmenorrhea improves
vitamin D levels. Although vitamin D or curcumin-piperine alone
could improve the clinical outcome and cytokines levels in SLE,
curcumin-piperine combined with vitamin D had the best outcome
in improving the disease activity and cytokines levels among
patients.
Can curcumin supplementation break the vicious cycle
of inflammation, oxidative stress, and uremia in patients
undergoing peritoneal dialysis?
Clinical
Nutrition ESPEN | February 2024
Curcumin supplementation for
twelve weeks attenuates lipid peroxidation and might reduce
uremic toxin in patients with chronic kidney disease undergoing
dialysis. Supplementation with 2.5 g of curcumin, three days a
week for 12 weeks, reduced the mRNA expression of nuclear factor
kappa B (NF-κB) and protein C- high-sensitivity reactive
(hs-CRP) in patients undergoing hemodialysis. In this
single-blind, randomised, controlled trial, we showed that a
12-week curcumin supplementation reduced the levels of MDA, a
standard marker of lipid peroxidation and showed a tendency to
reduce the pCS, a uremic toxin from gut microbiota, in patients
with chronic kidney disease undergoing dialysis. A review
based on twelve clinical trials comprising 631 patients with
chronic kidney disease showed that curcumin supplementation has
a favourable impact on oxidative stress, inflammation, and
proteinuria. Similarly, a recent study from our group showed
that curcumin supplementation for patients with chronic kidney
disease CKD undergoing hemodialysis significantly reduced NF-κB
mRNA expression and plasma levels of hs-CRP. Curcumin has
phenolic groups in its chemical structure, which has essential
antioxidant activity as it is a hydrogen donor, stabilising free
radical molecules directly. Indeed, curcumin has been tested
because of its antioxidant and anti-inflammatory effects in
patients with chronic kidney disease CKD. Our results showed
that curcumin supplementation may be a potential nutritional
strategy to help alleviate oxidative stress.
Effect of curcumin on malignant hepatocytes and
mitochondria studied using atomic force microscopy
Micron | February 2024
Curcumin, an active ingredient of
Curcuma longa, exerts anti-inflammatory, antioxidant and
anticancer effects on the liver (Reyes-Gordillo et al., 2017).
Most studies on the effects of curcumin on hepatoma cells have
focused on biological processes, including cell apoptosis, gene
expression, and the role of proteins (Darvesh et al., 2012).
Research has shown that curcumin induces cell apoptosis via the
mitochondrial pathway (Trujillo et al., 2014), while research on
curcumin in mitochondria has mainly focused on oxidation
reactions and protein expressions (Bai et al., 2022). The
chemical structure of curcumin is a lipophilicity polyphenol.
The viability of SMMC-7721 cells decreased with increasing
curcumin concentrations, and the change was statistically
significant when the curcumin concentration was higher.
A Review on the Extraction Process and Therapeutic
Activity of Curcumin on Diabetes Mellitus and Cancer
International Journal For Multidisciplinary Research | February
2024
Curcumin (diferuloylmethane), the active ingredient in
turmeric, has significant antioxidant, anti-inflammatory and
anti-cancer properties. Research (in vitro and in vivo) showed
that curcumin can also inhibits the activity of some signaling
molecules (such as transcription factors, various enzymes, such
as protein kinases) and can be modulated in this way
inflammatory process, gene expression and could possibly control
the effectiveness of curcumin for the treatment of many organ
diseases, mainly diabetes and its complications. Turmeric or
Curcuma Longa is a natural product whose medicinal
properties have been widely studied and a wide range of
therapeutic effects in various diseases, including
neurodegenerative, liver, kidney damage, cancer and diabetes
have been linked mainly to its curcuminoid content. Action of
curcumin or curcuminoids as a hypoglycemic agent or only as a
healing aid improve metabolic profile and improve
diabetes-related complications such as diabetes nephropathy and
cardiopathy are discussed. Curcumin has also been shown to be a
mediator of chemoresistance and radioresistance. Anticancer
effects have been observed in a number of clinical trials,
mainly a natural chemopreventive agent in colon and pancreatic
cancer, cervical neoplasia and Barrett's metaplasia. The potent
antiproliferative effects of curcumin, which interact with
multiple intracellular signaling pathways, may enhance the
antitumor effects of gemcitabine. Turmeric has also be widely
used as antioxidant, antiamyloid, antimicrobial, antitumor,
immune response modulating and neuroprotective effects. Curcumin
also had antidepressant properties by modulating the release of
serotonin and dopamine. Curcumin has been used as a dietary
supplement for centuries and is considered pharmacologically
safe. The multiple functions of curcumin that affect in a
chemopreventive and directly therapeutic way show that it could
be a potential anti-cancer drug. Curcumin promotes cell death in
a variety of animal and human cell lines, including leukemia,
melanoma, and breast, lung, colon, kidney, ovary, and liver
carcinomas. Curcumin promotes cell death in a variety of animal
and human cell lines, including leukemia, melanoma, and breast,
lung, colon, kidney, ovary, and liver carcinomas
The Use of Curcumin in the Treatment of Colorectal,
Breast, Lung, and Prostate Cancers: An In Vivo study Update
JLAR | January 2024
Studies have demonstrated
that curcumin can potentially treat various cancers. There is
evidence that curcumin has significant anti-cancer properties,
including tumor growth inhibition, metastasis inhibitory
activity, and angiogenesis. Several studies have demonstrated
the versatility and potential of curcumin in treating cancer.
Curcumin has considerable cancer treatment potential, based on
the in-vivostudies. Curcumin and its derivatives have had
bifunctional properties in the past two decades, such as
antioxidant and anti-inflammatory effects. The anti-cancer
properties of curcumin have been demonstrated in vitro
and in vivo at all stages of cancer growth, including
the disease's promotion and initiation. Curcumin treats a wide
range of diseases, including asthma, allergies, coughs,
bronchial hyperactivity, sinusitis, anorexia, coryza, and
hepatitis. Many studies show its anti-inflammatory, antioxidant,
anti-infectious, hepatoprotective, thrombosuppressive,
cardioprotective, chemopreventive, anti-arthritic, and
anticarcinogenic properties. Curcumin has also modulated several
molecular targets in the body. According to the combined
evidence presented in the included studies, curcumin is
potentially effective against various types of cancer,
highlighting its numerous anti-cancer properties. Researchers
have found that curcumin inhibits tumor growth, induces
apoptosis, suppresses angiogenesis, and modulates several
cellular signaling pathways against cancer. Considering its
broad spectrum of action, curcumin may be used as a
complementary treatment and an adjunct therapy for these common
types of cancer. As a result of this systematic review, curcumin
has the potential to be a promising anti-cancer agent against
colorectal, lung, prostate, and breast. It is clear that
curcumin has excellent potential as a cancer treatment agent,
and further study of its potential is warranted to achieve more
effective and holistic cancer treatments.
Protective role of curcumin in disease progression
from non-alcoholic fatty liver disease to hepatocellular
carcinoma: a meta-analysis
Frontiers in
Pharmacology | January 2024
Curcumin demonstrates a
significant improvement in key indicators across the stages of
NAFLD, liver fibrosis, and HCC. The research results reveal that
curcumin effectively hinders disease progression at each stage
by suppressing inflammation. Curcumin exerted hepatoprotective
effects in the dose range from 100 to 400 mg/kg and treatment
duration from 4 to 10 weeks. With excellent anti-inflammatory,
antioxidant, and anti-cancer properties, curcumin is expected to
be an effective treatment for all stages of liver disease. Based
on the results of the quantitative analysis evaluated in this
system, we can conclude that curcumin is applicable to a
spectrum of NAFLD–LF–HCC models and that most liver diseases
proceed based on the hepatic inflammatory microenvironment. More
attention should be paid to the anti-inflammatory effects of
curcumin. Curcumin has been shown to be effective in
inhibiting the progression of NAFLD–LF–HCC at doses of 100–400
mg/kg over a 4–8 weeks duration with significant
hepatoprotective effects, and its therapeutic mechanisms are
related to multiple pathways, including anti-inflammatory,
antioxidant, and apoptotic regulations which are regulated in
all stages of liver disease.
The Effect of Curcumin on the Gut-Brain Axis:
Therapeutic Implications
Journal of
Neurogastroenterology and Motility | January 2024
Several
types of nutrients, such as curcumin, have been proposed as
regulators of the dysbiotic state, and preclinical experiments
have suggested that curcumin is not only beneficial but also
safe. Curcumin possesses anti-inflammatory,
anti-atherosclerotic, neuroprotective and metabolic
disorder-modulatory effects. Indeed, the gut microbiota directly
interacts with curcumin to produce small catabolites that can be
absorbed through the intestinal wall, as confirmed by the high
concentration of curcumin in the gut after oral administration.
In addition, curcumin influences the gut microbiota by promoting
the growth of beneficial bacterial strains, improving microbial
richness and diversity as well as enhancing intestinal barrier
function. Curcumin also had beneficial effects on the gastric
tissue of diabetic gastroparesis model rats, exhibiting anti-gastroparetic
properties through improving ghrelin (a gut-brain peptide
hormone) expression, thereby balancing energy and promoting
gastrointestinal motility in the presence of oxidative stress.
Likewise, curcumin demonstrated a modulatory role on gastric
emptying via enhancing stem cell factor/c-kit signaling (through
reduction of oxidative stress) and the nuclear factor kappa B
cascade in the stomach of a diabetic gastroparesis rat model.
In fact, curcumin may be one of the non-invasive alternatives to
the invasive oral endoscopic gastric myotomy procedure. Curcumin
positively affects multiple pathways in Parkinson’s disease (PD)
treatment such as the inhibition of α-synuclein aggregation, an
increase in tyrosine hydroxylase, and reduction of N-acetylneuraminate
degradation, along with its influence on gut microbiota. Some in
vivo studies have indicated that the protective effect of
curcumin on neurodegenerative diseases and PD occurs through the
regulation of the gut microbiota by curcumin. Oral
administration of curcumin can alter the diversity of beneficial
or pathogenic bacteria accord- ing to the dose, duration of
treatment or formulation. In addition, curcumin and its
metabolites may have a direct impact on neurons, hormones,
blood, lymphatic vessels, and immune cells; thus, it is likely
that the direct and indirect effects of curcumin (gut-brain
axis) act together synergistically to modulate disease and
improve host health. Based on the beneficial effects of curcumin
on health-promotion, disease management, and its wide
application as a spice in daily food, this review has sought to
understand the interplay between curcumin and the gut-brain axis
in multiple diseases, mainly based on available in vivo
experimental models. The findings presented here indicate that
curcumin has promising potential, with acceptable efficacy, as a
regulator of the gut-brain axis in several dis- eases associated
with GM dysbiosis. In addition, curcumin can not only act as a
treatment but is also able to interact with intestinal
microflora in dysbiosis to target microbiota activation or
suppression, thereby enhancing its therapeutic effect through
the production of more active metabolites and better
pharmacokinetics.
Curcumin
Mitigates the High-Fat High-Sugar Diet-Induced Impairment of
Spatial Memory, Hepatic Metabolism, and the Alteration of the
Gut Microbiome in Alzheimer’s Disease-Induced (3xTg-AD) Mice
Nutrients | January 2024
Curcumin
enriched beneficial gut microbiota. The observed alteration in
these gut microbiota profiles suggests a potential crosswalk in
the liver and brain for regulating metabolic and cognitive
functions, particularly in the context of obesity-associated
cognitive disfunction, notably Alzheimer’s disease. Curcumin, a
major bioactive chemical constituent derived from turmeric, has
been found to exhibit a range of neuroprotective effects,
including the reduction in the amyloid burden,
neuroinflammation, oxidative stress, infection, and
inflammation. Previous findings also provided evidence of the
modulatory role of curcumin in mediating several targets of
metabolic diseases. Reduced plasma glucose and triglyceride and
improved β cell function and afamin levels were also evident
after curcumin consumption in some clinical trials. Our previous
studies have demonstrated the protective effects of curcumin on
metabolic dysfunctions such as body weight gain, liver fat
accumulation, and dysregulated insulin homeostasis in HFHSD-fed
middle-aged and old mice. Curcumin supplementation demonstrated
a notable impact on body weight gain in the 3xTg-AD mice
subjected to HFHSD. This aligned with the previous findings from
our studies and others, revealing a consistent reduction in body
weight in the mice following curcumin supplementation,
particularly under various metabolic challenges. Curcumin
supplementation effectively ameliorated these memory deficits,
as evidenced by an enhanced performance during the Y-maze test,
consistent with our prior finding. In conclusion, our study
demonstrated the multifaceted effects of curcumin on the body
weight, metabolic pathways, memory function, and gut microbiota
in 3xTg-AD mice under HFHSD conditions. The selective reduction
in body weight gain, improvements in memory function, and the
modulation of the metabolic pathways suggest the potential
therapeutic value of curcumin in mitigating Alzheimer’s-related
symptoms.
Curcumin Inhibits Bladder Cancer by Inhibiting
Invasion via AKT/MMP14 Pathway
Discovery
Medicine | January 2024
Curcumin could inhibit bladder cancer
by inhibiting invasion through the AKT/MMP14 pathway. The target
of curcumin for bladder cancer includes signal transducer and
activator of transcription 3 (STAT3), AKT, cyclin A2 (CCNA2),
epidermal growth factor receptor (EGFR), E1A binding protein
p300 (EP300) and MMP14. MMP14 was highly expressed in bladder
cancer than in normal tissues and was associated with a worse
prognosis (p < 0.05). Curcumin could inhibit the proliferation
and migration of bladder cancer cells.
Targeting endothelial cells with golden spice
curcumin: A promising therapy for cardiometabolic multimorbidity
Pharmacological Research | January 2024
Curcumin, a widely
used dietary supplement derived from the golden spice
Curcuma longa, has demonstrated remarkable potential in
treatment of CMM through its interaction with endothelial cells.
Numerous studies have identified various molecular targets of
curcumin (such as NF-κB/PI3K/AKT, MAPK/NF-κB/IL-1β, HO-1, NOs,
VEGF, ICAM-1 and ROS). These findings highlight the efficacy of
curcumin as a therapeutic agent against cardiometabolic
multimorbidity through the regulation of endothelial function.
Curcumin, derived from the golden spice Carcuma longa and widely
used as a dietary supplement, possesses anti-inflammatory,
analgesic, anti-angiogenic and anti-oxidative properties.
Several studies have demonstrated that the therapeutic effects
of curcumin against cardiometabolic multimorbidity were
attributed to its ability to target ECs. Studies have shown that
in patients with obesity, curcumin (oral, 1 g/d for 4 weeks)
could effectively regulate inflammation and immunity by reducing
levels of hs-CRP, IL-1β, IL-4, or VEGF, and decreased levels of
MCP-1, IL-6, and TNF-α in serum of metabolic syndrome (MS)
patients. Curcumin, a natural compound derived primarily from,
the rhizomes of Curcuma longa, possesses significant
pharmacological properties. Extensive studies have confirmed its
high oral safety. Additionally, our review has highlighted the
tremendous potential of curcumin in the treatments a majority of
cardiometabolic multimorbidities by the alleviation of
endothelial damages. Furthermore, research studies have
demonstrated the potential of curcumin in alleviating
endothelial inflammation, oxidative stress, and cellular
inflammation caused by diverse pathological conditions, such as
hyperglycemia, hyperlipidemia, hypertension, atherosclerosis,
cerebral ischemia, and myocardial infarction. In animal models
of obesity, MS, diabetes, and related complications, curcumin
was administered at a higher dose and for a longer duration of
300–400 mg/kg/day for 8–12 weeks. This dosage exceeds the amount
used to treat hypertension, atherosclerosis, and cerebral
ischemia, which falls within a dosing range of 100–200 mg/kg/day
for 4–6 weeks.
Examination
of the effect of curcumin supplementation on liver enzymes and
some physiological parameters in volleyball players
Revista de Gestão e Secretariado | January 2024
It has been observed that curcumin supplementation applied in
addition to volleyball training affects the lipid metabolism and
physiological parameters of the athletes. In line with this
information, we believe that the supplements to be applied in
addition to their routine training will positively affect the
athletic performance of the athletes. Curcumin has been
investigated for its anti-obesity, anti-inflammation,
anti-cancer, anti-angiogenesis, anti-diabetes, hepatoprotection,
radioprotection, and chemopreventive action. Curcumin is also
said to affect obesity and lipid metabolism via a number of
pathways, including energy metabolism regulation, inflammation
suppression, and angiogenesis. Turmeric has lately gained
widespread interest from researchers who have done studies
demonstrating that its therapeutic characteristics are linked to
pain relief, as well as the prevention and treatment of
cardiovascular, cancer, and other chronic diseases. Curcumin
administration has also been shown to improve physical activity
and sports performance in animal studies. Curcumin
supplementation is said to aid in muscle repair and
inflammation reduction, improve mitochondrial biogenesis, reduce
oxidative stress, and prevent fatigue and muscle damage. During
aerobic exercise (1 hour treadmill jog), there was a substantial
reduction in physical exercise-derived inflammation compared to
the pre-exercise moment in studies when turmeric supplementation
was used. Despite some inconsistent results, curcumin
administration appears to be useful for pain relief as well as
muscle damage mitigation by lowering serum CK. Nicol et al.
(2015) found that those treated with curcumin (5 g/day -5 days)
had less muscular pain and lower CK serum values (22-9%; ±
21-22%) at 24-and 48 hours. Curcumin supplementation for 8 weeks
resulted in substantial improvements in CRP, LDH, MDA, and VO2
max values in healthy young adult women in trials. It has been
observed that curcumin supplementation applied in addition to
volleyball training affects the lipid metabolism and
physiological parameters of the athletes. In line with this
information, the diversity of nutrients or compounds derived
from food factors or medicinal plants can be explored to
understand their possible effects on exercise physiology and the
different bioactivities that can be used for health promotion.
Profiling Inflammatory Biomarkers following Curcumin
Supplementation: An Umbrella Meta-Analysis of Randomized
Clinical Trials
Evidence-Based
Complementary and Alternative Medicine | January 2024
The
umbrella of meta-analysis suggests curcumin as a promising agent
in reducing inflammation as an adjunctive therapeutic approach
in diseases whose pathogenesis is related to a higher level of
inflammatory biomarkers. Curcumin is remarkable bioactive
polyphenol extracted from the rhizome of turmeric (Curcuma
longa). Curcumin has a wide range of medicinal effects such
as hepatoprotective, antimicrobial, anti-inflammatory,
antioxidant, and antitumor activities. The anti-inflammatory
feature of curcumin is mediated by several pathways. Intestinal
alkaline phosphatase, an endogenous antioxidant and
anti-inflammatory enzyme, is upregulated by curcumin. Moreover,
curcumin modulates inflammatory markers through nuclear
factor-erythroid factor 2-related factor 2 (NRF2∗)-Keap1
regulatory pathway. Given these effects, curcumin can have
regulatory effects on the level of inflammatory biomarkers such
as CRP, IL-6, and TNF-α. According to the US Food and Drug
Administration (FDA) report, curcumin has been considered as
“Generally Recognized as Safe” (GRAS) even at doses between 4000
and 8000 mg/day. The beneficial effect of curcumin on various
health conditions in some studies shows that the
anti-inflammatory effect of curcumin is not dependent on the
disease. Curcumin has a polyphenol nature; therefore, its
anti-inflammatory mechanisms may be due to its antioxidant
properties. Many properties of curcumin such as antioxidant,
anti-inflammatory, antimicrobial, and antimutagenic attribute to
the presence of hydroxyl and methoxy groups in the curcumin
structure. The present study shows that curcumin has reducing
effects on IL-6, CRP, and TNF-α levels. Therefore, curcumin can
be considered as a useful agent for longevity through decreasing
oxidative stress.
Anti-Inflammatory
Mechanisms of Curcumin and Its Metabolites in White Adipose
Tissue and Cultured Adipocytes
Nutrients |
January 2024
Overall, our in vivo and in vitro studies
demonstrate that curcumin alleviated diet-induced
obesity-associated inflammation. The plant-derived polyphenol
curcumin alleviates the inflammatory and metabolic effects of
obesity, in part, by reducing adipose tissue inflammation. Both
curcumin and its metabolites reduced LPS-induced adipocyte IL-6
secretion and mRNA levels. Proteomic analyses indicated that
curcumin upregulated EIF2 and mTOR signaling pathways. Overall,
curcumin exerted anti-inflammatory effects in adipocytes.
Previously, we reported that curcumin exerted protective
metabolic effects in diet-induced obese mice, independent of
changes in body weight. Curcumin’s effects may have been
mediated through its metabolic products, which are produced in
the gut, liver, or other tissues.
The
Effects of Curcumin on Neurodegenerative Diseases: a Systematic
Review
Journal of Herbal Medicine | January
2024
Curcumin has been considered in the therapeutic approach
to neurodegenerative diseases due to its relevant antioxidant
and anti-inflammatory properties. Adding curcumin to traditional
drug therapy appears promising and safe for treating
neurodegenerative diseases. Curcumin could be an option due to
its antioxidant, anti-inflammatory, and immunomodulatory
properties (Colaço et al., 2023, Grant et al., 2023, Marton et
al., 2022). The consumption of this plant or its derivatives,
such as curcumin, can improve the function and structure of
synapses by regulating proteins and delaying neuronal
dysfunction processes.
Antioxidant curcumin induces oxidative stress to
kill tumor cells (Review)
Oncology Letters
| January 2024
Curcumin is a plant polyphenol in turmeric
root and a potent antioxidant. Curcumin is a plant polyphenol in
the rhizome of turmeric and was classified as a third-generation
cancer chemopreventive agent by the National Cancer Institute.
Several studies have reported anticancer mechanisms mediated by
curcumin through the induction of elevated ROS. Curcumin has
anti-inflammatory, antibacterial, hepatoprotective and
anticancer properties, and its anticancer effects have been
reported in several tumor types. In melanoma, curcumin has been
reported to increase the ROS level and activate oxidative stress
in the cysteine asparaginase pathway, which causes tumor cell
death. Furthermore, curcumin-induced accumulation of ROS in
tumors to kill tumor cells has been noted in several studies, as
discussed in the present review. Curcumin is well tolerated by
humans. For example, a study that evaluated the toxicity of
curcumin in humans reported that subjects administered 8 mg/day
curcumin did not develop toxicity. Curcumin is a natural
compound that has been used for the treatment of numerous types
of diseases, such as Alzheimer's disease, fatty liver and
cancer. Of note, curcumin has a dual role in oncological and
non-oncologic diseases. Specifically, in non-neoplastic
diseases, curcumin is a potent antioxidant that attenuates
oxidative stress and mitochondrial damage. Conversely, in
tumors, curcumin binds to several enzymes and increases ROS
levels. These different effects may be the result of differences
in dosage. For instance, in a previous study on curcumin
treatment of drug-resistant tumor cells, a low dose of curcumin
showed no effect on antioxidant proteins, whereas a high dose
resulted in the inhibition of antioxidant proteins, thereby
increasing ROS levels. Furthermore, mitochondria may be a
potential target for high-dose curcumin. In addition, compared
with normal cells, proteins abnormally expressed in tumor cells,
such as GSH and HO-1, may be targeted by curcumin to cause
oxidative stress in tumor cells. In terms of autophagy,
curcumin has been reported to induce elevated ROS levels and the
appearance of autophagy markers and autophagosomes, causing
tumor cells to undergo autophagy. Curcumin achieves anticancer
effects by regulating the expression of Nrf2 and its downstream
target HO-1, inhibiting the expression of GPX4 and altering the
accumulation of intracellular iron and inducing the Fenton
reaction. Over the past two decades, the mechanisms by which
curcumin inhibits several types of tumor have been gradually
elucidated. In conclusion, curcumin may have the potential
to become a cutting-edge drug for the treatment of tumors and
other diseases.
Antioxidant, anti-inflammatory and epigenetic
potential of curcumin in Alzheimer's disease
BioFactors | January 2024
Curcumin - an integral component of
traditional medicine in numerous cultures worldwide - has
garnered interest as a promising Alzheimer's disease treatment.
Current research indicates that curcumin may exhibit therapeutic
potential in neurodegenerative pathologies, attributed to its
potent anti-inflammatory and antioxidant properties.
Additionally, curcumin and its derivatives have demonstrated an
ability to modulate cellular pathways via epigenetic mechanisms.
This article aims to raise awareness of the neuroprotective
properties of curcuminoids that could provide therapeutic
benefits in Alzheimer's disease. The paper provides a
comprehensive overview of the neuroprotective efficacy of
curcumin against signaling pathways that could be involved in
Alzheimer's disease and summarizes recent evidence of the
biological efficiency of curcumin in vivo. Curcumin is a
naturally occurring polyphenol found in turmeric, which has long
been utilized in traditional medicine for its anti-inflammatory
and antioxidant properties. Curcumin's anti-inflammatory
properties stem from its capability to inhibit multiple
proinflammatory signaling pathways mediated by nuclear factor
kappa B (NF-kB) and immune cell activation. Through this
modulation of inflammatory mediators, encompassing cytokines,
adhesion molecules, growth factors, and enzymes, curcumin holds
the potential for therapeutic benefits. Numerous studies have
investigated the therapeutic potential of curcumin against
various neurodegenerative diseases, including Alzheimer's
disease. Some evidence suggests that curcumin may be able to
counteract the formation of amyloid plaques. Additionally,
curcumin has been found to modulate Alzheimer's
disease-associated epigenetic changes by influencing methylation
patterns, microRNAs, and histone-modifying enzymes. Multiple
human studies suggest a potential of curcumin to modulate
Alzheimer's disease pathways in-vivo. Zhang et al.'s 2006
findings demonstrated in-vivo effectiveness of curcumin in
clearing amyloid deposits. It was observed that treatment of
macrophages of Alzheimer's disease patients with curcuminoids
notably enhanced the uptake of Aβ. Research by DiSilvestro et
al. extended these findings, investigating the effect of
curcumin on inflammatory pathways and Aβ clearance in healthy
middle-aged individuals. Daily administration of a low dose of
curcumin has exhibited potential in reducing plasma levels of Aβ
protein and augmenting antioxidant capacity by elevating the
levels of radical scavenging enzymes catalase and
myeloperoxidase. In a different study, a solid lipid formulation
of curcumin was observed to enhance cognitive performance,
alleviate fatigue, and mitigate the detrimental effects of
psychological stress. Further exploration into the therapeutic
potential of curcumin in Alzheimer's disease was initiated by
Baum et al.'s double-blind study in 2008. Their 6-month clinical
study confirmed the previously described advantageous effects of
curcumin in Alzheimer's disease, as such showing its potential
to promote disaggregation of Aβ and anti-inflammatory and
antioxidant responses. In 2020, Thota et al. demonstrated that
daily oral curcumin supplementation for 12 weeks could decrease
circulating levels of islet amyloid peptide (IAPP) and glycogen
synthase kinase-3 (GSK-3β), both implicated in insulin
resistance. Curcumin and its metabolites have been shown to play
a neuroprotective role, with the capacity to alter the
pathological sequelae that may lead to Alzheimer's disease.
Curcumin's demonstrated antioxidant and anti-inflammatory role,
along with its high safety profile, poses an intriguing
possibility in preventing and treating Alzheimer's disease.
Curcumin
in Alzheimer’s Disease and Depression: Therapeutic Potential and
Mechanisms of Action
Brazilian Archives of
Biology and Technology | January 2024
Curcumin is a
polyphenol present in Curcuma longa, a root used in Asian
cuisine for thousands of years, and it has several medicinal
properties, acting as an antioxidant, anti-inflammatory,
anticancer, among others. The data analyzed demonstrated that
curcumin supplementation is able to restore BDNF (Brain Derived
Neurotrophic Factor) levels in Alzheimer's disease and
depression, in addition to modulating monoamines and reducing
oxidative stress, inflammation, beta-amyloid aggregation, Tau
protein accumulation and aluminum neurotoxicity, improving their
symptoms. Curcumin has several medicinal properties as
antioxidant, anti-inflammatory, anti-HIV, antibacterial and
antitumor effect. In addition, curcumin is also used as a
therapeutic agent in inflammatory bowel disease, pancreatitis,
arthritis, some types of cancer [24], head trauma, anxiety,
Parkinson’s, depression, Alzheimer's disease, as well as acting
as BDNF restorer. Most of curcumin benefits can be attributed to
its anti-inflammatory action, obtained by the modulation of the
expression and production of enzymes such as cyclooxygenase-2
(COX-2), lipoxygenase and inducible nitric oxide synthase
(iNOS), and by the inhibition of inflammatory cytokines,
including interleukin and tumor necrosis factor alpha (TNF-α),
monocyte chemotactic protein (MCP), among others. In
neurodegenerative and neuropsychological diseases, curcumin's
role in restoring BDNF levels and, consequently, promoting
neurogenesis, is extremely important and may contribute to the
reversal of cognitive and mood disorders. After chronic curcumin
supplementation, subjects had a significant reduction in
depressive symptoms, with a reduction in the Hamilton Depression
scale and the Montgomery-Asberg scale. Curcumin supplementation
was also able to elevate plasma BDNF levels and reduce
inflammatory cytokines TNF-α and IL-1β, and salivary cortisol
concentrations, when compared to the placebo group. These
results suggest that supplementation with curcumin is able to
improve the action of selective serotonin reuptake inhibitor
class of antidepressants, such as escitalopram, mainly by
increasing BDNF levels, inhibiting proinflammatory cytokines and
reducing cortisol secretion. Therefore, there is strong evidence
of curcumin potential as a therapeutic agent in the
neuropsychological and neurodegenerative context, especially
when used in more bioavailable and high-quality formulations. In
summary, the data presented suggests that curcumin is able to
prevent and improve the symptoms of Alzheimer’s disease through
the improvement of cognitive and memory deficit by restoring
BDNF levels, reducing oxidative stress, inflammation,
beta-amyloid aggregation, Tau protein accumulation and aluminum
neurotoxicity. In Depression, curcumin treatment was also able
to improve its symptoms by restoring BDNF levels, modulating
monoamines and reducing inflammation. This study helps to
clarify the various mechanisms of action of curcumin in the
neurodegenerative / neuropsychological diseases, Alzheimer’s
disease and depression, suggesting it as a potential therapeutic
agent without any significant side effects.
Targeted therapies of curcumin focus on its
therapeutic benefits in cancers and human health: Molecular
signaling pathway-based approaches and future perspectives
Biomedicine & Pharmacotherapy | January 2024
Curcumin
modulates several biochemical pathways and targets involved in
cancer growth. Owing to its anti-inflammatory, antioxidant,
mutagenic, and antibacterial properties, curcumin has been
utilized as medication in Asian countries owing to their
antioxidant and anticancer activities. Additionally, it
has been shown to be advantageous for kidneys. Curcumin has
several medical uses, most of which are attributed to its
inflammatory and antioxidant properties. Curcumin has
anticancer, anti-inflammatory, antioxidant, and neuroprotective
properties, potentially managing diabetes, easing arthritis, and
potentially reducing inflammation in bowel diseases. Curcumin
can cure metabolic syndrome, anxiety, hyperlipidemia, and
oxidative and inflammatory diseases. Furthermore, a low dose can
still be beneficial for one's health, even if they have not been
diagnosed with a health condition. It has great potential as a
cancer treatment.
Curcumin
and breast cancer: therapeutic potential and mechanism in
multi-drug resistance
Cancer Genomics |
January 2024
The sensitizing effects of curcumin have been
studied in composition with chemotherapy drugs including
cisplatin,paclitaxel, doxorubicin, and 5-fluorouracil in BC
celllines such as MCF-7, MCF-7ADR, MDA-MB-231. Studies reported
that curcumin increases the sensitivity in the resistant cells
to chemotherapy drugs, which has apoptotic and growth inhibitory
effects. Also, curcumin could decrease multi-drug resistance
breast cancer through induction ofautophagy by down-regulate the
expression of CCAT1, PI3K/Akt/mTOR pathway. According to
the results of preclinical studies, curcumin administration can
inhibit the multi-drug resistance in breast cancer cells.
Analysis: Aaron Rodgers' remarkable recovery sets
standard for players who tear an Achilles tendon
The Independent | January 2024
Aaron Rodgers defied
conventional medicine by returning to practice just 77 days
after surgery for a torn Achilles tendon. Rodgers had a “speed
bridge” procedure that’s designed to expedite the recovery
process. He said Thursday that he attributes his progress to
working hard in rehab and a strict diet: “High levels of
curcumin, high levels of collagen and drinking freakin’ bone
broth every single day.” The soon-to-be 40-year-old Rodgers may
have cracked the code for an injury that’s typically
season-ending and normally takes players at least nine months to
recover. No professional athlete is known to have returned from
it in less than five months.
Curcumin
for Treating Breast Cancer: A Review
Pharmaceutics | January 2024
Curcumin, a phytochemical
derived from Curcuma longa (turmeric), has shown
substantial potential in inhibiting BC cell migration,
metastasis, and proliferation. Curcumin constitutes the primary
bioactive compound found in the plant Curcuma longa,
commonly known as turmeric. Curcumin, a natural compound found
in the turmeric plant Curcuma longa, is generally
considered safe when consumed in amounts commonly found in foods
and traditional herbal remedies. In 2021, the European Food
Safety Authority (EFSA) established an acceptable daily intake
of curcumin at 3 mg/kg body weight. For optimal pharmacological
effects, an oral dose of more than 8.0 g/day is often required.
Numerous clinical studies demonstrated that a daily intake of 12
g of curcumin is well tolerated and safe. Curcumin
unquestionably exhibits potential as an anticancer agent, with
relevance not only to breast cancer but also to lung cancer,
gastric cancer, and other malignancies.
Protective
role of curcumin in disease progression from non-alcoholic fatty
liver disease to hepatocellular carcinoma: a meta-analysis
Frontiers in Pharmacology | January 2024
Curcumin
demonstrates a significant improvement in key indicators across
the stages of NAFLD (Non-Alcoholic Fatty Liver Disease), liver
fibrosis, and HCC (Hepatocellular Carcinoma). The research
results reveal that curcumin effectively hinders disease
progression at each stage by suppressing inflammation. With
excellent anti-inflammatory, antioxidant, and anti-cancer
properties, curcumin is expected to be an effective treatment
for all stages of liver disease. This is the first meta-analysis
to correlate Non-Alcoholic Fatty Liver Disease, liver fibrosis,
and hepatocellular carcinoma and evaluate the therapeutic
effects of curcumin on systemic liver disease. The mechanisms of
curcumin in the inhibition of the transition from Non-Alcoholic
Fatty Liver Diseaseto HCC are multiple, well-established, and
multi-targeted. The phenotypes involved are mainly related to
oxidative stress, inflammation, and apoptosis. Based on the
results of the quantitative analysis evaluated in this system,
we can conclude that curcumin is applicable to a spectrum of
Non-Alcoholic Fatty Liver Disease–LF–HCC models and that most
liver diseases proceed based on the hepatic inflammatory
microenvironment. In the development of a range of liver
diseases, one of the most remarkable molecular changes driving
the Non-Alcoholic Fatty Liver Disease–LF–HCC axis is the NF-κB
signaling pathway, so anti-inflammatory processes play a crucial
role in all three disease stages. More attention should be paid
to the anti-inflammatory effects of curcumin. Curcumin
exerted hepatoprotective effects in the dose range from 100 to
400 mg/Kg and treatment duration from 4 to 10 weeks. The
mechanistic analysis reveals that curcumin primarily exerts its
hepatoprotective effects by modulating multiple signaling
pathways, including TLR4/NF-κB, Keap1/Nrf2, Bax/Bcl-2/Caspase 3,
and TGF-β/Smad3. In summary, curcumin has shown promising
therapeutic effects during the overall progression of
Non-Alcoholic Fatty Liver Disease-LF-HCC. It inhibited the pathological progression by
synergistic mechanisms related to multiple pathways including
anti-inflammatory, antioxidant, and apoptosis regulation.
Therapeutic effects of curcumin on
constipation-predominant irritable bowel syndrome is associated
with modulating gut microbiota and neurotransmitter
Frontiers in Microbiology | January 2024
In a
30-day randomized trial, in subjects with irritable bowel
syndrome, abdominal bloating can be successfully reduced with a
supplementation with curcumin (Giacosa et al., 2022). Previous
studies have shown that CUR has a significant effect on reducing
the levels of 5-HT in serum and colon (Yu et al., 2019).
Curcumin is the major active constituent of Curcuma
longa. Modern studies have demonstrated its potent
anti-inflammatory (Kocaadam and Şanlier, 2017), antioxidant (Ahangarpour
et al., 2019), and antidepressant (Chang et al., 2016) effects.
It has been found that treatment with curcumin can modulate 5-HT
levels, with treatment with curcumin significantly increasing
5-HT levels in the hippocampus, while decreasing 5-HT levels in
the colon in IBS models (Yu et al., 2015). Curcumin can interact
directly with the gut microbiota. Curcumin has been shown to
positively affect the gut microbiota and modulate microbiota
composition and function, further improving gut health (Ng et
al., 2018). These studies support the potential therapeutic
effect of curcumin on IBS-C through modulation of the gut
microbiota and neurotransmitters. The results of this study show
that curcumin is able to decrease the levels of HT, VIP and SP
and regulate the gut microbiota in rats with irritable bowel
syndrome-C, and then exert the therapeutic effect on irritable
bowel syndrome-C. The results suggest that curcumin may
represent a treatment option for irritable bowel syndrome-C
through modulation of the gut microbiota and relevant
neurotransmitters. Our results support the possibility of using
curcumin to treat irritable bowel syndrome-C patients.
The
impact of ginger and curcumin on diabetic nephropathy induced by
streptozotocin in rats
European Journal of
Translational and Clinical Medicine | January 2024
Curcumin
is a potent component in herbal medicine and is extensively
studied for various health conditions. Its primary compound,
polyphenol curcumin, exhibits powerful anti-inflammatory,
antioxidant, and anticarcinogenic properties. In diabetes
management, curcumin’s effectiveness lies in its interaction
with key molecules and pathways crucial in the disease’s
progression. Studies show curcumin’s ability to alleviate
insulin resistance, a factor in metabolic syndrome. Both ginger
and curcumin contain antioxidants that activate redox-sensitive
transcription factors, bolstering cellular antioxidant defenses.
Given the importance of dietary management in diabetes,
interventions using natural substances like ginger and curcumin
offer promising strategies to mitigate the renal complications
of DM. Ginger and curcumin extract contained the highest
phytochemical content and anti-oxidant activity (AOA). The
high glycoside content was recorded in curcumin. The high
terpene content was recorded in curcumin extract. The highly
alkaloid content was recorded in curcumin extract. Our results
demonstrated the anti-inflammatory and the antioxidant effects
of ginger and curcumin extracts, administered individually or in
combination. Our data have also shown that ginger and curcumin
extracts helped manage STZ-induced diabetic nephropathy and
oxidative stress via significant suppression of the NF-κB gene
expression. These extracts possess anti-inflammatory potential
by suppressing inflammatory cytokines and modulators through the
suppression of redox-based NF-κB activation.
Evaluating the potential of Vitamin D and curcumin
to alleviate inflammation and mitigate the progression of
osteoarthritis
PLoS One | January 2024
This study, for the first time, provides evidence of the
mitigating effect of Vitamin D and curcumin on PAR-2 mediated
inflammation. Nutraceuticals, such as Vitamin D and curcumin,
present potential therapeutic alternatives, offering
anti-inflammatory effects, potentially addressing osteoarthritis
inflammation. This study presents robust evidence that Vitamin D
and curcumin might represent a pioneering, natural, and
efficacious therapeutic strategy for managing osteoarthritis and
mitigating its related symptoms, specifically those exacerbated
by PAR-2 signaling. In conclusion, our study demonstrates that
both Vitamin D and curcumin can attenuate the pro-inflammatory
response in chondrocytes by inhibiting PAR-2 signaling, reducing
the expression of TNF α, IL 6, and IL 8, as well as the
RANKL/RANK system. Moreover, these bioceuticals also reduce IFN
γ expression, which amplifies the inflammatory events in OA.
These findings suggest that Vitamin D and curcumin have
potential therapeutic benefits in the management of
osteoarthritis.
The
impact of ginger and curcumin on diabetic nephropathy induced by
streptozotocin in rats
European Journal of
Translational and Clinical Medicine | January 2024
Our
results demonstrated the anti-inflammatory and the antioxidant
effects of ginger and curcumin extracts, administered
individually or in combination. Our data have also shown that
ginger and curcumin extracts helped manage STZ-induced diabetic
nephropathy and oxidative stress via significant suppression of
the NF-κB gene expression. These extracts possess
anti-inflammatory potential by suppressing inflammatory
cytokines and modulators through the suppression of redox-based
NF-κB activation. Our findings indicate that ginger and curcumin
extracts have therapeutic potential in mitigating functional and
structural alterations in the kidneys of diabetic rats, possibly
due to their anti-diabetic and anti-inflammatory properties.
Rats treated with combined ginger and curcumin extracts had
superior outcome in terms of more antioxidant activity, better
glycemia management and less DN-related kidney damage (reduced
albuminuria and less histological changes). Ginger and curcumin
are two well-known functional foods from the Zingiberaceae
family that have anti-inflammatory qualities. Curcumin is a
potent component in herbal medicine and is extensively studied
for various health conditions. Its primary compound, polyphenol
curcumin, exhibits powerful anti-inflammatory, antioxidant, and
anticarcinogenic properties. In diabetes management, curcumin’s
effectiveness lies in its interaction with key molecules and
pathways crucial in the disease’s progression. Studies show
curcumin’s ability to alleviate insulin resistance, a factor in
metabolic syndrome. Both ginger and curcumin contain
antioxidants that activate redox-sensitive transcription
factors, bolstering cellular antioxidant defenses. Given the
importance of dietary management in diabetes, interventions
using natural substances like ginger and curcumin offer
promising strategies to mitigate the renal complications of
diabetes.
Therapeutic
Potential of Curcumin, a Bioactive Compound of Turmeric, in
Prevention of Diabetes through the Modulation of Oxidative
Stress and Inflammation
Molecules | January
2024
In physiological and biochemical studies, it was found
that curcumin decreases glucose, creatinine, urea, and
inflammatory markers and increases antioxidant enzyme levels. In
addition, the histopathological findings revealed that curcumin
plays a significant role in the maintenance of renal tissue
architecture through the reduction in all pathological changes.
This study showed that curcumin has a vital role in the
regulation of the expression pattern of the IL-6 protein and
fibrosis. Based on biochemical and histopathological findings,
this study delivers a scientific suggestion that curcumin could
be a suitable remedy in the management of diabetes mellitus. The
novelty of the current study is that curcumin showed
anti-fibrotic potential by reducing collagen fiber deposition.
Curcumin treatment showed a significant decrease in parameters
and an increase in insulin level as compared to negative control
rats. Oral administration of curcumin significantly ameliorated
changes. Hence, based on biochemical and histopathological
findings, this study delivers a scientific suggestion that
curcumin could be a suitable remedy in the management of
diabetes mellitus. Curcumin, a yellow-colored compound, is
produced by plants of Curcuma longa species, and it is
chemically known as 1,7-bis(4-hydroxy-3-methoxyphenyl)-1,
6-heptadiene-3, 5-dione. It possesses antioxidant,
anti-inflammatory, anti-tumor, and other biological activities.
Curcumin is capable of exercising its antioxidant action via
scavenging a variety of hydrogen peroxide and nitric oxide (NO)
radicals and reactive oxygen species (ROS) as superoxide
radicals and by preventing lipid peroxidation. Moreover, several
in vitro as well as in vivo studies have described that curcumin
has potential for treating numerous inflammatory diseases.
Curcumin significantly reduced blood urea nitrogen, serum levels
of urea, and creatinine and simultaneously reduced
albumin/protein urea and increased creatinine clearance.
Further, it also prevented damage to renal tubules and the
thickness of the basement membrane. Curcumin treatment
efficiently counters diabetes-induced oxidative-stress-mediated
hepatic damage. Another finding reported that curcumin improved
the survival as well as the function of islet cells, with
reduced cell apoptosis in the islet of Langerhans and increased
insulin secretion in the STZ-induced diabetic model.
Exploring the Potential of Curcumin in Preserving
Telomere Length: A New Frontier in Cellular Aging and Health
Medium | January 2024
Curcumin has been valued for centuries
for its medicinal properties, particularly its potent
antioxidant and anti-inflammatory effects. Recent studies have
explored the potential of curcumin in influencing the length and
maintenance of telomeres, providing insights into its possible
role in combating cellular aging. Curcumin's role in preserving
telomere length largely hinges on its antioxidant properties.
Oxidative stress, a result of an imbalance between free radicals
and antioxidants in the body, is a key factor in the
acceleration of telomere shortening. Curcumin, with its strong
antioxidant capacity, helps neutralize these free radicals,
potentially reducing the rate of telomere shortening and thereby
promoting cellular longevity. In addition, the anti-inflammatory
properties of curcumin might help mitigate damage, thereby
slowing down the rate of telomere attrition. This could be
particularly significant in the context of chronic diseases and
age-related conditions where inflammation plays a pivotal role.
One of the most intriguing aspects of curcumin's relationship
with telomeres is its potential effect on the activity of
telomerase. Some studies have shown that curcumin might
stimulate telomerase activity, thus aiding in the maintenance
and even extension of telomere length. This suggests a possible
direct intervention in the cellular aging process. The
implications of curcumin's effects on telomeres extend to the
prevention and management of various diseases. Age-related
diseases like cancer, neurodegenerative diseases, and
cardiovascular problems have been linked to telomere length.
Therefore, by preserving telomere length, curcumin could
potentially play a role in mitigating these conditions. Its
antioxidant and anti-inflammatory properties, combined with its
potential to impact telomerase activity, make it a compound of
significant interest in the fields of aging and disease
prevention. In the realm of medicine, the exploration of
curcumin's role in telomere biology could lead to novel
therapeutic strategies. For instance, it could be employed as
part of a regimen for diseases where telomere shortening plays a
key role.
The
effect of curcumin on the necroptosis signaling pathway in colon
cancer cells
Bulletin of Biotechnology |
January 2024
Curcumin, a yellow compound isolated from the
turmeric plant, is important in preventing cancer. Studies have
shown that curcumin has an anticancer effect by driving cancer
cells into apoptosis, but studies showing its effect on
necroptosis are inconclusive. Consequently, the current data
clearly suggest that curcumin is a prominent driver of
necroptotic signaling-mediated colon cancer cell death.
Accumulating mass of indication suggests that curcumin has
antioxidant, anti-inflammatory, anti-bacterial, anti-diabetic,
and anti-cancer activities (Selvam et al. 2019). Curcumin has
shown to have significant impact on many signaling pathways in
cancer cells, which controls various cellular activities. The
anticancer activities of curcumin have also been shown in colon
cancer cells (Selvam et al. 2019). Curcumin has been
demonstrated to show anti-cancer activity in HT-19 colon cancer
cells by suppressing colony formation, cell viability, and DLEC1
promoter methylation (Guo et al. 2015). In addition, curcumin
has been shown to suppress the level of ATG5 (autophagy related
5) protein in HCT-116 cells, leading to the suppression of
autophagosome formation, cellular senescence, and cell cycle
arrest (Mosieniak et al. Bull Biotechnol (2023). In addition,
studies have shown that curcumin has anticancer activity in
other colon cancer cells such as Caco-2, HCT-15, and SW620 (Selvam
et al. 2019). Curcumin, which is extracted from the plant
Curcuma longa, is known to have numerous biological and
pharmacological activities. Curcumin has been reported to
stimulate cell death pathways such as apoptosis, autophagy, and
pyroptosis in many different cancer cells. In a study by
Blakemore et al., curcumin was shown to disrupt the cell cycle
progression by inducing G2/M cell cycle arrest in various colon
cancer cells (Blakemore et al. 2013). In addition, curcumin
treatment has been shown to cause abnormal mitotic spindle
formation and DNA damage. In addition, curcumin has been shown
to dose- dependently suppress cell proliferation. Bull
Biotechnol (2023). Remarkably, in our study, curcumin was shown
to stimulate necroptosis by increasing the expression of RIPK1,
RIPK3, and MLKL genes, especially in HT -29 colon cancer cells.
In conclusion, present findings strongly indicates that curcumin
is a significant driver of colon cancer cell death mediated by
necroptotic signaling.
Archived studies and
news on curcumin and turmeric
1984 - 2023
|
|
How may Curcumin
effect aging and longevity as an antiaging agent? |
Effect of Curcumin
on Vascular Aging
| Growing evidence indicates curcumin as a promising
antiaging agent. The effects of curcumin feeding have been
largely investigated in animal models, unanimously reporting a
suppression of intermediated oxidative stress and inflammation.
By chelating nitrogen dioxide (NO2), curcumin administration in
mice significantly attenuates nitric oxide- (NO-) associated
vascular endothelial dysfunction and generation of advanced
glycation end-products (AGEs), leading determinants of
age-related large elastic artery stiffening. As an additional
mechanism, curcumin fixes lysosomal membranes and reduces the
function of lysosomal acid hydrolases, thus preventing the
aberrant deposition of different connective tissue components in
aging endothelium. curcumin mitigated hypertrophy in the aging heart
via suppression of p300, the global transcription activator.
Beneficial effects of curcumin on vascular aging also concern
the development of age-related macular degeneration (AMD), one
of the most important causes of blindness in elderly. Curcumin
remarkably increases the viability of retinal pigment epithelial
cells (RPECs) modulating their proliferation apoptosis and OS.
Overall, those evidences suggest potential application of
curcumin as an innovative approach to AMD, as for other ocular
diseases (e.g., ocular dryness, conjunctivitis, uveitis,
pterygium, and glaucoma). Even curcumin has been found to
prevent the development of cataract in diabetic rats by
decreasing AGE accumulation and serum LPO. Curcumin reverses
those effects in cultured ECs, whereas in experimental models,
prolonged curcumin feeding decreased ROS generation and promoted
cerebrovascular endothelium-dependent relaxation, finally
leading to improved cerebrovascular function. Neuroprotective
effects of curcumin due to UCP2 overexpression suppression
especially target hippocampal neurogenesis in the CA1 area, thus
affecting spatial learning and memory. Curcumin also prevents
detrimental effects of chronic cerebral hypoperfusion by
maintaining cholesterol homeostasis. Curcumin also contributes
to maintain cholesterol homeostasis, otherwise upset by chronic
cerebral ischemia. Indeed, curcumin promotes cholesterol efflux.
Effect of Curcumin on Longevity and Lifespan | Curcumin was
shown to increase the fecundity, reproductive lifespan, and
child viability of D. melanogaster. It has been shown
that curcumin supplementation of D. melanogaster
elevated the developmental duration and longevity of adult
Drosophila possibly through epigenetic programming of the
pace of life. Curcumin increased longevity was observed in two
distinctive strains of D. melanogaste as a result of the delayed
expression of aging genes, improved locomotion, and
chemoprevention as well. Curcumin was also shown to reduce OS,
DNA damage, and number of mutagenic phenotypes induced via
high-dose ionizing irradiation. Also, in vivo experiments on
curcumin -fed diets (0.5 and 1.0 mg/g of diet) were effective in
extending the average lifespan in both females (6.2% and 25.8%,
respectively) and males (15.5% and 12.6%, respectively). Also,
in C. elegans, curcumin effectively improves lifespan
and aging by lowering intracellular ROS and lipofuscin. The
effects of curcumin on C. elegans longevity are
manifested by body size and pharyngeal pumping rate. This
evidence indicates that curcumin would exert its effects
independently of the Age-1-DAF-16 pathway but rather through
other constituents of the IIS pathway. With regard to cognitive
impairment, the in vivo experiment demonstrated that curcumin
can improve learning and memory also reducing Aβ plaque
formation in the context of Alzheimer disease.
D.
melanogaster is a promising animal model for research in
AD. By increasing amyloid fibril conversion, curcumin reduces
the generation of prefibrillar/oligomeric species of Aβ,
ultimately protecting against neurotoxicity. The human β-amyloid
precursor cleavage enzyme (BACE-1) is another critical enzyme
targeted by curcumin in the D. melanogaster model of
Alzheimer disease.
Effect of Curcumin on Cell Senescence | The
antiaging effect of curcumin does not rely on delayed cellular
senescence. As reported by Banji et al., curcumin (40 mg/kg) and
piperine (12 mg/kg), especially when combined, counteract
D-gal-induced senescence in male Wistar rats by targeting OS and
lipofuscin deposition, finally leading to higher hippocampal
volume and function with improved spatial memory and
serotoninergic signaling. Another study even reported how
long-time curcumin therapy may progressively reverse cognitive
dysfunction in D-gal-induced senescent mice by delaying the
aging process and improving cognitive functions and locomotor
activity, as well as restoring the mitochondrial enzyme complex
function curcumin. In a recent study, curcumin supplementation
rejuvenates senescence-associated changes in thymus among
D-gal-induced senescent mice through promotion of proliferating
cells, preventing cells from apoptosis, and enhancing the
transcription of the autoimmune regulator. Curcumin feeding
(50 mg/kg) was also tested in senescence-accelerated mouse prone
mice resulting in increased hippocampal SOD activity as well as
upregulation of p-calcium/calmodulin-dependent kinase II.
Overall, these findings suggest a role of curcumin in improving
cognitive difficulties and the expression of hippocampal
plasticity-associated proteins. With regard to vascular
function, curcumin administration significantly mitigated
premature senescence in HUVECs, characterized by a reduction of
senescence-related β-galactosidase-positive cells, cell
division, levels of senescence-related protein p21 RNA, OS, and
apoptosis. Curcumin is also associated with enhanced eNOS
phosphorylation and NO generation, in addition to upregulating
Sirt1 transcription, translation, and enzymatic activity. In
light of these mechanisms, diets containing curcumin were
demonstrated to significantly extend mean lifespan in
male C57BL/6 mice and delayed the OS-caused premature
senescence. As recently demonstrated, Sirt1 signaling also
mediates the anti-inflammatory effects of curcumin in C57BL/6
mice fed with high fat diet in addition to improved myocardial
structure and function in streptozocin-induced diabetic mice fed
with THC (120 mg/kg/d). Even more recently, it has been
hypothesized that the antiaging effect of curcumin may rely on
the control of core clock genes. Curcumin treatment in middle
aged male Wistar rats restored the phase and daily pulse.
Moreover, it has been shown that curcumin mitigated mouse
ovarian aging, upgraded embryonic development, promoted oocyte
maturation and fertilization via improvement of ovarian
hormones, and elevated the amounts of SIRT1 and 3 genes as well
as attenuation of aging-associated oxidative stress and cell
death. Besides, curcumin can reduce oxidative stress,
inflammation status, and lipofuscin deposition in aged rat
liver. |
|
How may Curcumin work against Neurological and neurodegenerative
disorders such as Alzheimer's disease, Parkinson's disease,
Multiple Sclerosis, Huntington’s Disease, and cognitive decline
or impairment? |
Effect of Curcumin on Alzheimer's
Disease | Evidence has accumulated that curcumin has
neuroprotective properties and is a candidate for the treatment
of Alzheimer’s disease. In an Alzheimer’s disease transgenic
mouse, curcumin decreased oxidative stress and repaired amyloid
pathology. Antioxidant and anti-inflammatory features of
curcumin helped to minimize the manifestation of Alzheimer’s
disease, which is characterized by inflammation and oxidation.
Recent studies have indicated that curcumin treatment can
promote the decomposition of β-amyloid in brain tissues and
prevent the aberrant production and accumulation of β-amyloid,
which reduces the hyperphosphorylation of tau protein and
effectively prevents the degeneration and injury of brain
neurons. Curcumin has protective effects for several risk
factors of neurodegeneration and is used in the treatment of
Alzheimer's disease as well. In vivo studies show the beneficial
effects of curcumin on cognition with a dose-dependent manner
that higher dosages is more effective as compared to lower
dosages. Based on the preclinical findings, curcumin stabilizes/prevents cognitive decline in
Alzheimer's disease. A literature review was conducted to verify
studies that evaluated the effects of curcumin supplementation
on neurodegenerative / psychological disorders such as
Alzheimer's and depression in animal and human models, and their
respective mechanisms of action. Scholar google, ScienceDirect
and PubMed electronic databases were consulted up to September
2021. In the search, the following descriptor combinations were
used in the English-language databases: “BDNF”, “Neurogenesis”,
“Curcumin BDNF”, “Curcumin Bioavailability”, “Curcumin
Depression”, “Curcumin Depression BDNF”, “Curcumin Depression
Inflammation”, “Curcumin Serotonin Depression”, “Depression
BDNF”, “Depression Brain Size”, “Depression mechanism”,
“Depression Medication”, “Depression Neurogenesis”, “Polyphenol
Depression”, “Polyphenol Bioavailability”, “Alzheimer's”,
“Alzheimer's BDNF”, “Alzheimer's Curcumin”, “Curcumin
Alzheimer's BDNF”, “Alzheimer's Inflammation”, “Alzheimer's
Oxidation”, "Curcumin Tau protein", "Alzheimer Metals",
"Alzheimer Neurogenesis", "Exercise BDNF", "Calorie restriction
BDNF ”,“ Omega 3 BDNF ”. In their review, Pluta and colleagues focus on the role
and mechanisms of curcumin in inhibiting ischemia/reperfusion
brain injury and potential therapeutic strategies in the
treatment of ischemic brain damage of the Alzheimer’s disease
phenotype. Comparably, Ferreira and colleagues also delineate
neuroprotective characteristics by summarizing what is known
about the role of curcumin on transthyretin amyloidosis.
According to previous reports, curcumin modulates abnormal
transthyretin (TTR) aggregation and inhibits its deposition in
the tissue. As the gut–brain axis is linked to
neurodegeneration, curcumin exerts neuroprotective effect
against neurodegenerative disorders by restoring the intestinal
barrier function and a healthy gut microbiome. These findings
highlight the importance of neuroprotective effect of curcumin
against brain damage by regulating both inflammation and
oxidative stress. This is consistent with previous findings
where curcumin was shown to reduce significantly the mRNA
expression of NF-kB and TLR4 and showed protective effects
against glutamate neurotoxicity. Studies have shown that
curcumin has a therapeutic effect on Alzheimer’s disease by
several molecular mechanisms, including decreasing oxidative
damage and constraining the creation of the Aβ fibrils in vitro.
The anti-inflammation effects of curcumin as a food additive
were evaluated in the APPSw mice (Alzheimer-like model) at
several doses. The results have indicated that low-dose curcumin
(160 ppm) reduced GFAP, which is an astrocytic marker associated
with inflammatory processes. Furthermore, the effect of curcumin
on spatial memory (an Alzheimer’s disease symptom) in
Alzheimer’s disease rat models has shown that curcumin
significantly decreases GFAP mRNA in hippocampal astrocytes,
which improves the spatial memory in the Alzheimer’s disease rat
model. Ambegaokar et al. reported that the inhibition property
of curcumin is dose and time-dependent. For example, curcumin
concentrations of 15–30 μM are more effective for short trials
(<24 h), while its concentrations of 5–15 μM are better suited
for longer periods (4–6 days). These data suggest that curcumin
may be more effective in preventing AD in low doses if used for
long periods. Accumulating data show that Aβ can increase the
expression of COX-2, IL-1, and IL-6, while decreasing the
peroxisome proliferator-activated receptor-gamma (PPARγ) in
amyloid-beta protein precursor transgenic mice, and curcumin can
inhibit this function in amyloid-beta-treated astrocytes. Most
of the literature about curcumin indicates that this spice has
especially strong properties against AD. For instance, the
incidence of Alzheimer’s disease among Indian people (who
regularly consume these spices) is very low when compared with
the reported incidence in Western countries. Only 0.7% of 70–79
years old people in India are affected by AD; however, about
3.1% of Americans in this age range are also affected. Up to
now, the potential anti-amyloid therapeutic methods for
Alzheimer’s disease treatment have been focused on the amyloid
cascade theory, on which the use of Aβ vaccines and
metal-complexing agents is based. Strimpakos et al. reported
that curcumin has anti-amyloidogenic properties, thus acting
against AD-induced Aβ fibrils in vitro and improving cognitive
functioning in vivo. Interestingly, another study
analyzing the curcumin-mediated neuroprotective effects on brain
aging induced by d-galactose in in vitro and in vivo models
revealed an anti-aging effect through regulating neuronal loss,
apoptosis in D-galactose induced brain aging, and anti-oxidant
enzyme expression. Furthermore, curcumin improved neuronal
length and cellular senescence down-regulated expression of p16
and p21 and upregulated expression of antioxidant enzymes,
including SOD-1, GPX-1, and catalase. Administration of curcumin
ameliorated the cognitive impairment and suppressed apoptosis in
the cerebral cortex by downregulating Bax and poly (ADP-ribose)
polymerase expression and increasing Bcl-2 expression [86]. In
neurodegenerative diseases, such as AD, PD, ALS, microglia play
an important role by inducing oxidative stress, redox imbalance
and neuroinflammation. The activated microglia are represented
by M1 (pro-inflammatory) and M2 (anti-inflammatory) functional
phenotypes based on the surface molecules and cytokine
expression profiles. Different natural products show therapeutic
properties on microglia and consequent prevent neurodegenerative
diseases; they act by inhibition of microglia polarization and
production of inflammatory mediators. In microglia, curcumin
acts on different molecular targets. Curcumin inhibited
LPS-induced NF-kB and activator protein-1 (AP-1) DNA bindings in
BV2 microglial cells decreasing inflammatory mediators.
Peroxisome proliferation-activated receptor-γ (PPARγ) is a
transcription factor and nuclear receptor protein that regulates
inflammatory responses in microglia, astrocytes and when is
activated, PPARγ suppresses the production of proinflammatory
cytokines and inflammatory pathways by binding the peroxisome
proliferator response element. Curcumin activates PPARγ
which reduces NF-κB cytokine production in a mouse model of AD,
in rat hippocampal primary cell lines and primary astrocytes.
Moreover, our group has found that curcumin suppresses LPS
induced inflammatory response in microglia cells by down
regulation of PI3K/Akt and JAK/STAT/SOCS signaling pathway. In
addition, curcumin induces anti-inflammatory mediators, such as
HO-1/NRF-2 consequently reducing oxidative stress and
neuroinflammation. Curcumin treatment improved neuron loss and
degeneration, while also inhibited cellular senescence and
oxidative stress by upregulating antioxidant enzyme expression
in RA-induced SY5Y cells. In line with the findings described
above, the protective effect of curcumin against cognitive
impairment has been demonstrated in diabetes mellitus/chronical
cerebral hyperperfusion-induced cognitive deficit model.
Moreover, curcumin treatment attenuated the neuronal death and
suppressed neuroinflammation induced by microglial activation.
These protective effects involved the modulation of triggering
receptor expressed on myeloid cells 2 (TREM2)/TLR4/NF-kB
pathway. Curcumin treatment reduced nod-like receptor protein 3
(NLRP3) dependent pyroptosis. Since NLRP3-dependent pyroptosis
has been reported to be involved in the progression of
neurodegenerative diseases, this result suggests that curcumin
may be useful as pharmacological strategy for neurodegenerative
diseases. Further studies are needed for better understanding of
curcumin’s promising effects in preventing the neuronal loss and
cognition-decline related to aging. The pleiotropic activities of curcumin provide
multiple ways to tackle TTR pathophysiology, through direct
interaction of curcumin with TTR, or indirect effects affecting
signaling pathways associated with TTR amyloid fibril formation
and clearance. Bielak-Zmijewska and coworkers summarize
scientific data on curcumin’s ability to postpone progression of
age-related diseases in which cellular senescence is directly
involved. They furthermore point out that curcumin causes
elongation of the lifespan of model organisms and alleviates
aging symptoms. In addition, they discuss thoroughly curcumin’s
ability to modulate cellular senescence. Common brain disorders, including depression and Alzheimer’s disease, have
been linked to diminished levels of an important neurologic growth hormone
called brain-derived neurotrophic factor. Reports suggest Curcumin has
neuroprotective action in Alzheimer’s disease, major depression,
epilepsy, and other neurodegenerative disorders. The hippocampus
region of brain is associated with memory and cognition. Studies
have shown that hippocampus undergoes structural and biochemical
changes with normal aging that results in age-related
deterioration in hippocampus-dependent cognition. Curcumin has
been found to ameliorate age-related memory deficits in aged
mice. In elderly, regular curcumin intake improves cognitive
function and ameliorates age-related spatial memory deficits. An
Australian study, in the
Journal of Psychopharmacology in 2015,
found that curcumin improved attention and working memory and reduced mental
fatigue in older people who took it for four weeks, compared to a placebo. Several studies have shown that
curcumin, the active medicinal compound in turmeric, can increase levels of
BDNF in the human brain and therefore delay or even reverse a range of
common neurological disorders. One of the main drivers of this process is
brain-derived neurotrophic factor (BDNF), which is a type of
growth hormone that functions in your brain (20). Many common
brain disorders have been linked to decreased levels of this
hormone, including depression and Alzheimer's disease.
Interestingly, curcumin can increase brain levels of BDNF. By
doing this, it may be effective in delaying or even reversing
many brain diseases and age-related decreases in brain function.
It may also improve memory and make you smarter, which seems
logical given its effects on BDNF levels. However, controlled
studies in people are needed to confirm this. In addition, scientists are beginning to
suspect that the neurologic powers of curcumin don’t just stop there, with
research suggesting that this compound may improve memory and increase
cognitive capacity. Curcumin, thanks to its wide range of effects,
seems to help the brain resist buildup of harmful plaque in brains with
Alzheimer's. A
study in
the Annals of Indian Academy of Neurology explored curcumin's potential
for use in the treatment for Alzheimer's disease. Some of the key points
included: Curcumin may help the macrophages, which play an important role in
our immune system, clear the amyloid plaques found in Alzheimer's disease.
Curcumin has anti-proliferative actions on microglia. Microglia are immune
cells of the central nervous system that become active in response to any
number of stressors on the body. However, if the microglia have been
stimulated to react too often, they become hyper-reactive, which can trigger
system-wide inflammation that can be difficult to stop. Curcumin has
powerful antioxidant and anti-inflammatory properties. "Overall, curcumin
decreases the main chemical for inflammation and the transcription of
inflammatory cytokines …The exposure to curcumin also impaired the
production of pro-inflammatory cytokines (IL-1, IL-6 and TNF-)." As chronic
neuro-inflammation is considered one of the major factors in the development
of Alzheimer's, it's possible too that curcumin may help in the treatment of
other inflammatory disorders. Researchers found that Curcumin
not only reduces oxidative damage and inflammation, but also
reduces amyloid accumulation and synaptic marker loss and promotes amyloid
phagocytosis and clearance. Curcumin worked to prevent synaptic marker and
cognitive deficits caused by amyloid peptide infusion and abeta oligomer
toxicity in vitro, and may help the immune system clear the
brain of amyloid beta, which forms the plaques found in Alzheimer's disease. Clinical trials are in progress at UCLA with Curcumin
for Alzheimer's. In the
Alzheimer’s Disease Anti-Inflammatory Prevention Trial, researched showed that
reducing inflammation has positive effects on patients with Alzheimer’s.
Curcumin significantly lowered several inflammation markers, in addition to
reducing plaque on the brain (a sign of Alzheimer’s) by 43 to 50 percent.
The effect of curcumin (turmeric) on Alzheimer's disease: An overview
Neuroprotective activity has also been shown in curcumin. In Alzheimer’s
disease (AD), a peptide called β-amyloid (Aβ peptide) aggregates into
oligomers and fibrils and forms deposits known as amyloid (or senile)
plaques outside neurons in the hippocampus and cerebral cortex of patients.
Another feature of AD is the accumulation of intracellular neurofibrillary
tangles formed by phosphorylated Tau protein. Abnormal microglial
activation, oxidative stress, and neuronal death are also associated with
the progression of the disease. Curcumin has been found to inhibit Aβ fibril
formation and extension and to destabilize preformed fibrils
in vitro. Metal chelation by curcumin might interfere with metal ion
(Cu2+/Zn2+)-induced Aβ aggregation. Curcumin might also affect the
trafficking of Aβ peptide precursor (APP) and the generation of Aβ peptides
from APP. Abnormally activated microglia and hypertrophic astrocytes around
amyloid plaques in AD brains release cytotoxic molecules, such as
proinflammatory cytokines and ROS, which enhance Aβ formation and deposition
and further damage neurons. Curcumin was found to reduce the inflammatory
response triggered by Aβ peptide-induced microglial activation and increase
neuronal cell survival. When injected into the carotid artery of a
transgenic mouse model of AD, curcumin was found to cross the blood-brain
barrier, bind to amyloid plaques, and block the formation of Aβ oligomers
and fibrils. In other animal models of AD, dietary curcumin decreased
biomarkers of inflammation and oxidative damage, increased Aβ peptide
clearance by macrophages, dismantled amyloid plaques in the brain,
stimulated neuronal cell growth in the hippocampus, and improved Aβ-induced
memory deficits. As a result of promising findings in animal models. a few
recent clinical trials have examined the effect of oral curcumin
supplementation on cognition in healthy older adults and AD patients. A significant reduction in mental
fatigue and higher levels of calmness and contentedness following cognitive
test sessions were observed in individuals who consumed curcumin (either
acutely or chronically) compared to the placebo group. Additionally, the
results of cognitive ability tests suggested that curcumin treatment had
limited benefits on cognitive function, as shown by better scores in
measures of sustained attention and working memory compared to placebo. The
results of a six-month trial in 27 patients with AD found that oral
supplementation with up to 4 g/day of curcumin - containing all three major
curcuminoids - was safe. Curcumin also helps inhibit
plaque that research has linked to neuron damage in the brain
and a sign of the disease. There may be good news on the horizon
because curcumin has been shown to cross the blood-brain
barrier. It’s known that inflammation and oxidative damage play
a role in Alzheimer's disease, and curcumin has beneficial
effects on both. In addition, a key feature of Alzheimer's
disease is a buildup of protein tangles called amyloid plaques.
Studies show that curcumin can help clear these plaques.
Alzheimer’s disease (AD) is a chronic neurodegenerative disease
characterized by the presence of hyperphosphorylated tau protein
in neurofibrillary tangles, selective neuronal loss, progressive
memory and cognitive impairment (Campbell and Gowran 2007). The
molecular pathogenesis of AD involves extracellular deposition
of beta-amyloid (Ab) peptides in the hippocampus and curcumin
is known to reduce Alzheimer’s pathology (Serafini et al. 2017)
possibly due to its anti-aggregatory properties (Cole, Teter,
and Frautschy 2007). In a clinical study, curcumin
administration (1 or 4 g, 6 months trial) significantly
increased the levels of antioxidant vitamin E without inducing
any adverse events in patients with AD (Baum et al. 2008). In
preclinical studies, curcumin is known to reduce Aboligomer and
fibril formation (Yang et al. 2005; Xiong et al. 2011), inhibit
the neurotoxicity of Abin the brain (Jiang et al. 2012; Sun,
Zhao, and Hu 2013), suppress Ab-induced inflammation (Lim et al.
2001; Lu et al. 2014) and markedly reduce the levels of IL-
1b(Griffin et al. 2006) and inducible nitric oxide synthase
(iNOS) (Begum et al. 2008) in transgenic mouse brain. Several
studies demonstrated dose-dependent neuroprotective effect of
curcumin against Ab-induced toxicity. Curcumin exhibited
anti-aggregatory effect against Ab plaque formation by metal
chelation (Huang et al. 2004; Tamagno et al. 2005), anti-oxidant
effects (Hamaguchi et al. 2009), cholesterol lowering effects
(Fassbender et al. 2001; Refolo et al. 2001), inhibition of
presenilin-2 and/or by increasing degrading enzymes such as
insulin-degrading enzyme and neprilysin (Wang et al. 2014).
Curcumin potentiate heat shock proteins production in response
to cellular stress, which protects neuronal cells from Ab
neurotoxicity and prevent Ab aggregation and accumulation
(Scapagnini et al. 2006; Ohtsuka and Suzuki 2000; Cummings et
al. 2001).
Effect of Curcumin on Parkinson’s Disease |
Dietary curcumin is an important candidate in the prevention or
treatment of Parkinson’s disease.
Curcumin is suggested to be an effective therapeutic and
nutraceutical agent for Parkinson’s diseasetreatment.
Interestingly, curcumin was found to inhibit the synthesis of
MOA-B enzyme (Khatri and Juvekar, 2016), which would lead to an
increase in the level and availability of DA in the brain.
Neuroprotective effects of curcumin in a 6-hydroxydopmin e
animal model of Parkinson’s disease (El Nebrisi et al., 2020)
indicated an increase in the survival of striatal TH fibers and
SNpc neurons, decreased abnormal turning behavior, and exerted
neuroprotective properties. These findings provide evidence that
α7-nicotinic acetylcholine receptors could be a potential
therapeutic target and curcumin would be the first natural
source that is found to modulate nicotinic receptors in
Parkinson’s disease.
Recent evidence indicates
decreased superoxide dismutase 1 (SOD1) expression in reactive
astrocytes in the damaged substantia nigra, thus leading to
inflammation and oxidative stress that contribute to the
degeneration of dopaminergic neurons in Parkinson’s disease.
Curcumin, through the preservation of SOD1 expression in
reactive striatal astrocytes in hemiparkinsonian mice, has
anti-inflammatory properties. Gui et al. showed that curcumin,
through the inhibition of CYP2E1 (the cytochrome P450 2E1)
expression and its activity in reducing ROS and maleic
dialdehyde in astrocytes, leads to protection of the
mesencephalic astrocytes against LPS-induced toxicities. These
results indicate that curcumin could affect the metabolism of
several compounds in the CNS and provide evidence for the
therapeutic approach in Parkinson’s disease using curcumin at
low concentration. Studies show that the oral administration of
curcumin (150 mg/kg/day for a week) in mouse models of
Parkinson’s disease reversed GFAP and inducible nitric oxide
synthase protein expression and also decreased proinflammatory
cytokine in the striatum, suggesting that curcumin can improve
motor performance in a mouse model of Parkinson’s disease. In
addition, curcumin, through the Bcl-2-mitochondria-ROS-inducible
nitric oxide synthase pathway, can protect against MPP+
(1-methyl-4-phenylpyridinium)- and MPTP−
(1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine)-induced
apoptosis in PC12 cells. Curcumin can significantly inhibit
NF-κβ translocation and activation in astrocytes. In one study, chronic curcumin administration (50,
100 or 200 mg/kg, p.o., for 3 weeks) significantly ameliorated
behavioral alterations like locomotor activity and
motor-coordination in mouse model of Parkinson’s disease. In the
similar study, curcumin administration reduced oxidative damage
and mitochondrial dysfunction in brain homogenate by reducing
AChE activity. Curcumin administration decreased malondialdehyde
(MDA) and nitrite while increased superoxide dismutase (SOD),
catalase (CAT) and reduced glutathione (GSH) levels in the brain
homogenate of rotenone induced mouse model of Parkinson’s
disease (Khatri and Juvekar 2016). It has been demonstrated that
curcumin administration alleviate motor dysfunction and increase
tyrosine hydroxylase activity in rotenone induced Parkinson’s
disease rat model. Curcumin administration phosphorylates Nrf-2
and Akt thereby attenuated oxidative damage of dopaminergic
neuron (Cui, Li, and Zhu 2016). Moreover, dietary curcumin
supplementation 0.5% or 2.0% (w/w) attenuated
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induced
neurotoxicity in mice via increasing the expression of glial
cell line-derived neurotrophic factor and TGF-b1 in
nigrostriatal dopaminergic system and thus slowing the
progression of Parkinson’s disease (He et al. 2015b). Curcumin
administration increased monoaminergic neurotransmitters such as
norepinephrine and dopamine in hippocampal homogenate and
alleviated hippocampal damage in 6-hydroxydopamine induced
Parkinson’s disease in rat. In addition, curcumin treatment
upregulated the expression of BDNF, TrkB and PI3K in the
hippocampus (Yang et al. 2014). Curcumin treatment (200 mg/kg,
for 1 week) significantly attenuated loss of tyrosine
hydroxylase, sustained SOD1 level and diminished activation of
microglia and astrocytes in the striatum.
Effect of
Curcumin on Cognitive
Impairment | Curcumin has been reported to improve
neuropsychological functions. curcumin has several inhibitory
effects on combining aging and Alzheimer’s disease
pathophysiology, such as the suppression of amyloid precursor
protein (APP) and Aβ synthesis and the overexpression of ApoE
and Nrf2 gene, as well as the prohibition of p-mTOR and p-NF-κB.
Curcumin prevents D-gal-induced brain aging and cognitive
impairment through increments of antioxidant enzymes and
inhibition of apoptosis. Beneficial effects of curcumin on
mental abilities and functional capacities are associated with a
LPO reduction in brain tissue, especially in the hippocampal
area. Curcumin improves the redox state in this area and
prevents the decline of hippocampal long-term potentiation by
maintaining synapse input specificity. Recently, Olesen et al.
described that the dysfunction of synaptic mitochondria of the
hippocampus causing memory loss during aging. They showed that
curcumin feeding significantly improved integration and activity
of the synaptic mitochondrial of the hippocampus, inhibiting
mitochondrial swelling and enhancing the production of synapses
surrounding the mitochondria in mice.
Effect of
Curcumin on Multiple Sclerosis and Amyotrophic Lateral Sclerosis
| Curcumin was studied to determine if it could help ALS
patients, particularly those with bulbar involvement, survive
longer (Ahmadi et al., 2018). Curcumin therapy reduced the
development of ALS and oxidative damage in a double-blind
therapeutic trial (Chico et al., 2018). Recent studies suggest that curcumin, through reduced MMP-9
enzyme activity and decreased release of IL-6 in the astrocyte
population of CNS, might beneficially cause anti-inflammatory
responses in neurodegenerative diseases, such as multiple
sclerosis. Curcumin represents some potential for treatments of
various autoimmune diseases related to Th17 cells including MS.
Curcumin, through interfering with protein kinase C activity and
Ca2+ entry, can eliminate both PMA and thapsigargin-induced ROS
generation by the dose-dependent manner. Curcumin can prevent
the production of H2O2 and NO; the free radicals produced by
macrophages and astrocytes in vitro. In EAE, curcumin has
important roles in lymphocyte proliferation inhibition,
reductions of IL-17 production by Th17 cells, and Toll-like
receptors 4 and 9 (TLR 4 and 9) downregulations. Xie et al.
reported that in EAE mice or rats, curcumin shrinks inflammatory
cells, including Th17 cells, and hinders its infiltration and
differentiation in the CNS. Curcumin has the promising potential
for treating multiple sclerosis. Curcumin delivering to the
animal models through intraperitoneal injection or oral
administration leads to NF-κβ pathway activation decreasing in
rat and mouse microglial cell cultures and also in the rat
brain. Furthermore, curcumin can also reduce NF-κβ activation in
human cell lines and can neutralize ROS in vitro and can
stimulate the Nrf2-ARE pathway similarly in the brain and
skeletal muscles of mice and also in isolated rat astrocytes.
SOD1-misfolded and -aggregated proteins in the motor neurons
have an important role in disease pathogenesis, and its
targeting treatment can decrease ALS progression in animal
models. As previously shown, curcumin can constrain SOD1
aggregation in vitro. Curcumin through gene expression can also
stimulate the clearance of the aggregated protein in PALS and
Alzheimer’s disease blood cells. Up to the present time, several
studies confirm that regimens’ treatment can cause motor neuron
enhancement (although there are several different descriptions
for these improvements). Furthermore, a small pilot trial
revealed some advantage of curcumin in PALS.
Effect
of Curcumin on Huntington’s Disease | Studies using
established yeast models showed that curcumin inhibits mHTT
aggregation, by acting through endosome-sorting complexes
required for transport machinery and also destabilizes preformed
aggregates. Curcumin, by downregulation of Vps36, a component of
the endosome-sorting complexes required for transport-II
complex, prevents recruitment of misfolded protein to the
perivascular compartment, thus inhibiting the formation of large
aggregates. The amyloid-binding ability and anti-amyloid
properties of curcumin, along with its ease of oral
administration, make it an attractive therapeutic candidate for
several neurodegenerative diseases. |
| How may Curcumin work against diabetes? |
|
Because of its anti-inflammatory
property, curcumin represents a promising therapeutic option for
Type 2 Diabetes. Curcuminoids have been demonstrated in diabetes
mellitus type 2 patients to improve insulin resistance, reduce
glucose and insulin levels, enhance adiponectin secretion, and
lower levels of leptin, resistin, interleukin (IL-6, IL-1β), and
TNF-α (Hajavi et al., 2017). Curcumin and its three
derivatives (dimethoxy curcumin, bisdemethoxycurcumin, and
diacetyl curcumin) were reported for their antioxidant
capabilities (Faizal et al., 2009). Curcumin’s ability to decrease blood sugar
levels in human patients was first reported in 1972. A male
patient who had diabetes for 16 years ingested 5 g of curcumin
over a period, after which his fasting blood sugar decreased
from 140 to 70 mg/dl. Ingestion of curcumin along with insulin
synergistically reduced the blood sugar level. Furthermore, when
the insulin dosage was decreased to the minimum, the
anti-diabetic effect of curcumin was persistent. Interestingly,
when the ingestion of curcumin and turmeric was discontinued for
a week, random blood sugar levels increased to 140 mg/dl.
Therefore, ingestion of a daily 5-g dose of curcumin was
resumed, which promptly reduced the fasting blood sugar level to
110 mg/dl. Blood urea in this patient after 3 months of turmeric
therapy was 20 to 22, and the patient’s electrocardiogram was
normal. Turmeric therapy was not associated with any palpable
adverse effects; rather, the beneficial effects of turmeric as a
good appetite stimulant and effective laxative were observed.
More recently, a randomized, double-blind, placebo-controlled
clinical trial assessed the efficacy of curcumin in delaying
development of Type 2 Diabetes in the prediabetes population.
After 9 months of treatment, 16.4% of participants in the
placebo group were diagnosed with Type 2 Diabetes, whereas
none were diagnosed with Type 2 Diabetes in the
curcumin-treated group (Fig. 6a). In addition, the participants
of curcumin-treated group showed a better overall function of β
cells, with higher HOMA-β and lower C-peptide levels. The
curcumin-treated participants also exhibited a lower level of
HOMA-IR and higher adiponectin when compared with the placebo
group. The authors of this study concluded that the curcumin may
be beneficial in a prediabetes population.
Curcumin has been shown to equal in effectiveness to the drug metformin
in the management of diabetes, but without negative side effects. In the
study curcumin was shown to lower blood glucose levels and reverse insulin
resistance by suppressing glucose production in the liver. Among those
verging on type 2 diabetes, curcumin capsules
seem to help stall the onset of the disease. The study that returned
these results found that while a little more than 16% of people taking a
placebo wound up with a diabetes diagnosis, no one taking curcumin received
one. A
clinical trial from Thailand, published in
Diabetes Care in
2012, found that people with prediabetes who took curcumin for nine months
had improved function of insulin-producing cells in the pancreas, along with
a significantly reduced risk of developing type 2 diabetes. Other studies
suggest that curcumin can improve insulin sensitivity. Another study found curcumin
improved metabolic
function and reduced the risk of plaque buildup in the arteries of
type-2 diabetes patients. Curcumin also acts as an anti-diabetic and
antioxidant in patients with
type-1 diabetes. Curcumin acts directly on liver cells to help
prevent them from becoming fatty, and studies have concluded that Curcumin may
have an anti-diabetic effect by decreasing serum fatty acid through the
promotion of fatty acid oxidation and utilization. Curcumin also works directly on pancreatic beta cells
to help them produce insulin normally. By helping the liver and the pancreas,
Curcumin is taking stress off the two most important organs whose function
declines before the onset of type 2 diabetes. Curcumin also influences key
hormones, supports major body organs, and regulates inflammatory signaling all
in ways that help correct or prevent metabolic problems. Curcumin helps lower
inappropriately high levels of leptin (reducing leptin resistance) while
boosting the all-important levels of the adiponectin
(which lowers insulin resistance). Curcumin also helps activate the fat-burning
gene signal PPAR gamma, which also helps to make more new, metabolically-fit fat
cells. Curcumin directly reduces major inflammatory events from occurring inside
white adipose tissue (tumor necrosis factor alpha, interleukin-6, and monocyte
chemotactic protein-1). By lowering such inflammation, the source of
overweight-induced disease is targeted. Oxidative stress and inflammation have
been implicated in the pathogenesis of type 2 diabetes mellitus
and related vascular complications. A large body of preclinical
evidence suggests that the antioxidant, anti-inflammatory, and
glucose-lowering activities of curcumin and its analogs may be
useful in the prevention and/or treatment of type 2 diabetes. In
a nine-month, randomized, double-blind, placebo-controlled study
in 237 subjects with impaired glucose tolerance (pre-diabetes),
no progression to overt diabetes was reported with a daily
ingestion of a mixture of curcuminoids (0.5 g), while 16.4% of
placebo-treated participants developed diabetes. In addition,
curcumin supplementation was shown to reduce insulin resistance
and improve measures of pancreatic β-cell function and glucose
tolerance. Supplemental curcumin was found to be as effective as
lipid-lowering drug atorvastatin (10 mg/day) in reducing
circulating markers of oxidative stress (malondialdehyde) and
inflammation (endothelin-1, TNFα, IL-6) and in improving
endothelial function. Another randomized controlled trial also
reported that oral curcumin supplementation (1.5 g/day) for six
months improved endothelial function, insulin sensitivity, and
metabolic markers associated with atherogenesis (plasma
triglycerides, visceral fat, total body fat) in participants
with type 2 diabetes. Finally, in a two-month randomized,
double-blind, placebo-controlled study in 40 individuals with
type 2 diabetic nephropathy (kidney disease), daily curcumin
ingestion (66.3 mg) significantly reduced urinary concentrations
of proteins and inflammation markers (TGF-β, IL-8), suggesting
that curcumin might be helpful with slowing the progression of
kidney damage and preventing kidney failure. The research on
curcumin suggests it can work as a hypoglycemic agent—lowering
and helping control blood glucose (blood sugar) levels in people
with type 2 diabetes. This can ultimately prevent those with the
disease from developing other serious health complications
associated with diabetes, such as neuropathy (damage to the
nervous system) and nephropathy (kidney disease). A study
published in the journal Biochemistry and Biophysical Research
Community explored how curcumin might be valuable in treating
diabetes, finding that it activates AMPK (which increases
glucose uptake) and suppresses gluconeogenic gene expression
(which suppresses glucose production in the liver) in hepatoma
cells. Interestingly, they found curcumin to be 500 times to
100,000 times (in the form known as tetrahydrocurcuminoids(THC))
more potent than metformin in activating AMPK and its downstream
target acetyl-CoA carboxylase. Diabetic neuropathy is a type of
neuronal damage, associated with chronic diabetes, characterized
by demyelination and deterioration of nerve fibers, alterations
in the micro- vasculature and loss of sensory fibers that leads
to pain, foot ulcers, amputations, depression, phobias,
anorexia, loss of memory and reduction in complex reasoning
skills (Patel and Udayabanu 2013). |
|
Curcumin
treatment (50 mg/kg, for 8 weeks) upregulated BDNF in frontal
cortex and hippocampus alongside reduced oxidative damage in the
hippocampus of diabetic db/db mice (Franco-Robles et al. 2014).
Curcumin administration significantly increased Na (þ) -K (þ)
-ATP activity, reduced lactate dehydrogenase (LDH) activity and
lactic acid content as well as stimulates Ca (þ) -Mg (þ) -ATP
activity in brain homogenate of alloxan induced diabetic mice.
In addition, curcumin administration ameliorated energy
metabolism in the brain homogenate of diabetic mice (Miao,
Cheng, and Li 2015). Curcumin administration (60 mg/kg, p.o.,
for two weeks) downregulated the expression of glucose
transporter (GLUT) type 3, muscarinic receptor type 3,
a7-nicotinic receptor and AChE in brain- stem and cortex of
streptozotocin induced diabetic rats. In addition, it reduced
the expression level of insulin receptor and choline
acetyltransferase in brainstem. Curcumin treatment upregulated
the gene expression of choline acetyltransferase, SOD and
insulin receptor in cortex. It is known to upregulate the
expression level of muscarinic cholinergic receptor 1 in
brainstem and cerebral cortex (Kumar et al. 2013) as well as
attenuate cognitive deficits in streptozotocin induced diabetic
rats (Kumar et al. 2011). Curcumin treatment (60 mg/kg, p.o.,
for 15 days) downregulated the expression level of dopaminergic
D1 and D2 receptor in the cortex. In addition, curcumin
administration significantly upregulated dopaminergic D1
receptor and downregulated D2 receptor in the cerebellum of
diabetic rodents. Curcumin treatment upregulated phospholipase C
and transcription factor cAMP response element-binding protein
expression in the cerebellum and cortex of streptozotocin
induced diabetic rats resulting in amelioration of emotional
and cognitive performance (Kumar et al. 2010). Curcumin
administration (60 mg/kg, p.o., for 16 days) upregulated the
glutamate decarboxylase while downregulated Bax, caspase 3 and
caspase 8 expressions in the cerebral cortex. In addition,
curcumin administration attenuated NMDA and AMPA receptor
mediated oxidative stress and excitotoxicity in the cerebral
cortex of streptozotocin induced diabetic rats (Jayanarayanan et
al. 2013). Curcumin supplemented (0.5%) with animal’s diet
decreased b-d-glucuronidase activity (Chougala et al. 2012),
nitric oxide level, total oxidant status, MDA level and
oxidative stress index. Diabetes mellitus, commonly referred to
as diabetes, is a chronic metabolic disorder characterized by
hyperglycemia, glycosuria, negative nitrogen balance, polydipsia
and sometimes ketonemia. In a randomized, double-blind,
placebo-controlled trial, oral curcumin extract supplementation
(three capsules per day, each curcumin capsule has curcuminoid
content of 250 mg) for nine months ameliorated b-cell function,
lowered C-peptide and increased homeostasis model assessment-b,
reduced insulin resistance and increased the adiponectin level
in type 2 diabetic subjects as compared to placebo group
(Chuengsamarn et al. 2012). In another clinical study, curcumin
administration lowered the level of HbA1c and fasting blood
glucose as well as partially reduced LDL-cholesterol and body
mass index in diabetic subjects (Rahimi et al. 2016). A recent
meta-analysis revealed that, curcumin or combined curcuminoids
supplementation effectively lowered the level of fasting blood
glucose in individuals with some degree of dysglycemia. In
addition, isolated curcumin supplementation significantly
decreased HbA1c as compared to placebo and suggested its
beneficial role as adjuvant in the treatment of dysglycemic
patients (de Melo, Dos Santos, and Bueno 2018). In animal study,
curcumin administration is reported to reduce glucose
intolerance through induction of glucagon-like peptide-1
secretion. In addition, curcumin administration is known to
reduce insulin resistance by downregulating phosphorylation of
IRS-1 serine residue and upregulating phosphorylation of IRS-1
tyrosine in the skeletal muscle of rats fed with high fructose.
Curcumin treatment also reduced glucose intolerance,
hyperinsulinemia and homeostasis model assessment-insulin
resistance (HOMA-IR) level. Curcumin treatment decreased C
reactive protein and TNF-levels besides downregulated the
protein kinase theta (PKCh) and COX-2 protein expressions.
Additionally, curcumin significantly downregulated extracellular
kinase 1/2 (ERK 1/2) and p38 protein expressions in skeletal
muscle. Further, curcumin treatment ameliorated the activity of
GPx and attenuated the activation of inflammatory cascades
(Maithilikarpagaselvi et al. 2016). Curcumin treatment
significantly reduced systolic blood pressure, LDL-cholesterol,
triglycerides, aspartate transaminase (AST), alanine
transaminase (ALT), total cholesterol, glycemia, total oxidative
status, MDA and nitrative stress. A recent study demonstrated
that, curcumin administration (100 mg/kg, p.o., daily for 8
weeks) attenuated splenic damage and improved immunity in
streptozotocin-induced diabetic rats via antioxidant,
anti-inflammatory and anti-apoptotic mechanisms (Rashid et
al. 2017). Curcumin treatment is known to attenuate diabetes and
its associated complications like liver disease, adipocyte
dysfunctions, pancreatic beta cell dysfunction, vascular
dysfunction, nephropathy, neuropathy, retinopathy etc. (Zhang et
al. 2013b). In cell culture studies, curcumin treatment
suppressed palmitate-mediated insulin resistance, inhibited the
ubiquitin-proteasome system, reduced the endoplasmic reticulum
(ER) protein aggregation and activated the autophagy signaling
in human umbilical vein endothelial cells (Ye et al. 2017). The
suggested anti-diabetic mechanisms of curcumin effects are
ameliorating b-cell dysfunction, insulin signaling, glucagon
like peptide-1 secretion, and reducing glucose intolerance,
hyperglycemia, hyperinsulinemia, HOMA-IR level, hyperlipidemia,
islet apoptosis and necrosis etc. Therefore, these finding
demonstrate that curcumin supplementation in diabetic population
may be beneficial. |
| How may Curcumin work against
CANCER? |
In recent
years, in-depth studies of cancer progression have revealed that
curcumin suppresses tumors by interfering with all aspects of
tumor progression, which is the action of some of the most
promising anticancer drugs. First, at the root of cancer
progression, curcumin has been shown to elevate the
ubiquitination level of TAZ that increases proteasome-degrading
TAZ protein, thereby activating the hippo pathway and negatively
regulating cancer stem cell function. Additionally, curcumin
significantly impedes the self-healing of circulating cancer
stem cells, limiting stem cell metastasis. Curcumin also alters
the expression of more than 700 genes linked to carcinoma
development, such as those involved in DNA recovery or
associated with the cell cycle, cell proliferation, or
metastasis in NCI-H460 human lung cancer cells. Researchers
revealed that curcumin not only changes the expression of many
genes, but also alters signaling pathways. Through further
investigation, it was found that those curcumin-altered genes
induce cell death and control extracellular matrix receptors,
repressing NSCLC cell proliferation and migration. These
observations indicate that curcumin governs NSCLC tumor growth
and exhibits cytotoxic mechanisms at the genetic level. Curcumin
possesses various biological activities, such as anticancer
effects on various cancers, such as breast, liver, lung, gastric
and prostate cancers. The anticancer effects of curcumin have
been extensively studied in different cancers, such as breast,
lung, colorectal, head and neck, gastric, bladder, prostate,
thyroid, liver, ovarian, oral, pancreatic, cervical, tongue and
brain cancers. Because of its multitargeting activities, curcumin
has exhibited activities against numerous cancer types in human
clinical trials. Studies have demonstrated that curcumin can
suppress the proliferation of cancer cells by interfering with
the cell cycle. Specifically, it induces cell cycle arrest at
the G2/M phase in several cancer types, modulating the level of
cyclin-dependent kinases (CDKs) and cyclins through the
increased expression of CDK inhibitors. Furthermore, curcumin
induces apoptosis through both intrinsic and extrinsic pathways.
Curcumin enhances the expression of pro-apoptotic proteins such
as Bax, Bak, PUMA, Bim, and Noxa and death receptors such as
TRAIL-R1/DR4 and TRAIL-R2/DR5. In addition, curcumin decreases
the levels of anti-apoptotic proteins like Bcl-2, Bcl-XL, survin,
and XIAP. This shift in the balance of apoptotic regulators
facilitates the release of cytochrome c from mitochondria and
activates caspases, leading to programmed cell death. Curcumin,
a polyphenolic compound derived from turmeric, exerts its
anticancer effects through multiple mechanisms. These include
the inhibition of cell proliferation and the induction of
apoptosis via cell cycle arrest and the modulation of apoptotic
proteins. Curcumin suppresses the activity of key transcription
factors like NF-κB, STAT3, and AP-1 and interferes with critical
signal transduction pathways such as PI3K/Akt/mTOR and MAPK/ERK.
Additionally, curcumin inhibits angiogenesis and metastasis by
downregulating VEGF, VEGFR2, and matrix metalloproteinases
(MMPs). Epigenetic modifications through the inhibition of DNA
methyltransferases (DNMTs) and histone deacetylases (HDACs)
further contribute to its anticancer properties. Finally,
curcumin alters mitochondrial energy metabolism and reduces
oxidative stress by inhibiting FoF1-ATP synthase, thereby
impacting ATP production and reactive oxygen species (ROS)
generation, which are crucial for cancer cell growth and
proliferation. Curcumin’s anticancer efficacy is also attributed
to its ability to inhibit nuclear factor kappa B (NF-κB), a
transcription factor that plays a pivotal role in cancer cell
survival, proliferation, and metastasis. NF-κB is often
constitutively active in various cancers, promoting the
expression of genes involved in inflammation, cell survival, and
angiogenesis. Curcumin suppresses NF-κB activation by inhibiting
IκB kinase (IKK), thereby preventing the phosphorylation and
degradation of IκBα, an inhibitor of NF-κB, and reducing the
transcription of NF-κB target. In addition to NF-κB, curcumin
also modulates other transcription factors such as STAT3 and
AP-1. By inhibiting these factors, curcumin reduces the
expression of genes involved in cell proliferation and survival,
contributing to its anticancer properties. Curcumin’s impact on
various signal transduction pathways further elucidates its
multifaceted anticancer effects. It has been shown to interfere
with the PI3K/Akt/mTOR pathway, a critical signaling axis for
cell growth and survival. Curcumin inhibits the phosphorylation
of Akt, leading to the suppression of downstream targets
involved in cell proliferation and survival. Additionally,
curcumin affects the MAPK/ERK pathway, which is implicated in
cell differentiation, proliferation, and apoptosis. By
inhibiting this pathway, curcumin can reduce cancer cell growth
and induce apoptosis. Curcumin also exerts anti-angiogenic and
anti-metastatic effects, which are crucial for limiting tumor
growth and spread. Curcumin inhibits angiogenesis by
downregulating the expression of vascular endothelial growth
factor (VEGF) and its receptor VEGFR2. This inhibition prevents
the proliferation and migration of endothelial cells, thereby
reducing blood vessel formation. Moreover, curcumin inhibits
metastasis by modulating the expression of matrix
metalloproteinases (MMPs), enzymes involved in the degradation
of the extracellular matrix, a key step in cancer cell invasion
and metastasis. Curcumin downregulates MMP-2 and MMP-9, thereby
impairing the invasive capabilities of cancer cells. Also, it
inhibits the chemokine CXCL12/CXCR4 axis, whose activation is
involved in tumor epithelial–mesenchymal transition (EMT),
cancer cell motility, and metastasis. Emerging evidence suggests
that curcumin can also exert its anticancer effects through
epigenetic modifications. These modifications include the
inhibition of DNA methyltransferases (DNMTs) and histone
deacetylases (HDACs), which play a role in gene expression
regulation. By modulating these epigenetic factors, curcumin can
reactivate tumor suppressor genes and inhibit oncogenes,
contributing to its anticancer activity. Although the energy
metabolism of cancer cells appears to be supported more by
anaerobic glycolysis (the Warburg effect), mitochondria play a
pivotal role in cancer cell physiology, driving both energy
production and the biosynthetic processes essential for rapid
proliferation. Several studies reported that cancer cells often
exhibit altered mitochondrial function, characterized by
enhanced oxidative phosphorylation (OXPHOS) and increased
mitochondrial biogenesis. These adaptations support the high
metabolic demands of tumorigenesis, since mitochondrial function
reprogramming can confer resistance to chemotherapy and
contribute to metastatic potential. Beyond energy production,
mitochondria play a pivotal role in cancer cell growth and
proliferation, primarily through the generation of reactive
oxygen species (ROS). A mild ROS concentration induces the
activation of signaling pathways such as MAPK, PI3K/Akt, and
NF-κB, which are involved in cell proliferation and survival.
Moreover, oxidative stress can induce the expression of growth
factors and cytokines, enhancing tumor progression and
metastasis. In this scenario, curcumin appears to be one of the
most promising molecules to modulate OxPhos activity and the
related oxidative stress production. The literature reports that
curcumin directly inhibits the activity of FoF1 -ATP synthase
(ATP synthase), as it binds to the F1 moiety through its
4′hydroxy groups and a β-diketone, reducing the available energy
to support the proliferation and growth of cancer cells. In
addition, ATP synthase inhibition not only impacts ATP
production but also modulates oxidative damage. In coupling
conditions, ATP synthase reduction slows down electron transport
chain (ETC) function and the relative ROS production, switching
off the proliferation signaling associated with the pro-oxidant
environment. By contrast, when mitochondria are damaged,
curcumin concurs with the oxidative stress increment. Probably the first indication of curcumin’s
anticancer activities in human participants was shownby
Kuttan and co-workers, who conducted a clinical trial involving
62 patients with external cancerous lesions. Curcumin was found
to produce remarkable symptomatic relief as evidenced by
reductions in smell, itching, lesion size, and pain. Kuttan and
his colleague’s work was the first to demonstrate curcumin’s
anti-cancer potential in both in vitro and in vivo experimental
models (Kuttan et al., 1985). Curcumin activates DNA damage
response, laying the foundation for the therapeutic use of these
nutraceuticals in prostate cancer chemoprevention (Horie, 2012).
The general anti-carcinogenic effect of curcumin involves
mechanisms like induction of apoptosis and inhibition of
cell-cycle progression in rat aortic smooth muscle cells (Chen
and Huang, 1998). The antiproliferative effect is regulated
partly through hindrance of protein tyrosine kinase activity and
c-myc mRNA expression, while the apoptotic effect may partly be
mediated via preventing the functioning of protein tyrosine
kinase, protein kinase C, and expressions of c-myc mRNA and
bcl-2 mRNA (Chen and Huang, 1998). Curcumin inhibits the
transcription factor NF-κB (Figure 6) and various downstream
gene products like c-myc, Bcl-2, COX-2, nitric oxide synthase
(NOS), Cyclin D1, TNF-α, ILs, and matrix metallopeptidase 9
(MMP-9) and has anti-proliferative activities in a diversity of
malignancies. Curcumin, either alone or in combination with other agents, has
demonstrated potential against colorectal cancer, pancreatic
cancer, breast cancer, prostate cancer, multiple myeloma, lung
cancer, oral cancer, and head and neck squamous cell carcinoma. Curcumin was found to exert its anticancer activities
in many different types of cancer cells by regulating a variety of signaling
pathways. Curcumin induces cell death in numerous animal and human
cell lines, including leukemia, melanoma, and carcinomas of the
breast, lung, colon, kidney, ovaries and liver. It appears to
function by caspasedependent and independent (mitochondrial)
mechanisms, which are associated with the presence and absence
of p53. Curcumin has been studied as a beneficial herb in cancer
treatment and been found to affect cancer growth, development
and spread at the molecular level. Studies have shown that it
can contribute to the death of cancerous cells and reduce
angiogenesis (growth of new blood vessels in tumors) and
metastasis (spread of cancer). Multiple studies indicate that
curcumin can reduce the growth of cancerous cells in the
laboratory and inhibit the growth of tumors in test animals.
There is also evidence that it may prevent cancer from occurring
in the first place, especially cancers of the digestive system
like colorectal cancer.
Curcumin is capable of inhibiting the growth
of cancer cells in skin, oral, intestinal, and colon cancers.
Animal models show that not only does curcumin block growth of
cancer cells in these models, but it also increases the number
of cancer-fighting enzymes in the system. A 2011 study
works to quantify the prohibitive properties of curcumin on
cancer cells in head and neck squamous cell carcinomas. This
type of cancer is the 6th most commonly-diagnosed cancer in the
United States. That study showed curcumin not only works as a
treatment for squamous cell carcinomas with incredibly promising
results, but it has also been shown to demonstrate powerful
anti-cancer properties. Part of the excitement surrounding the
potential anti-cancer benefits of curcumin revolves around the
safety of use of the compound. It is considered
pharmacologically safe, which means there are no known drug
interactions or specific reactions among patients, making it
extremely well-tolerated. For more evidence that turmeric with
curcumin in particular being a powerful anti-cancer compound, we
need only look at the rates of cancer in parts of the world
where turmeric is consumed in higher quantities. Over the years, cancer research has
examined the role curcumin plays in treating this disease.
Curcumin is antimutagenic as it potentially helps to prevent new
cancers that are caused by chemotherapy or radiation therapy
used to treat existing cancers. It effectively inhibits
metastasis (uncontrolled spread) of melanoma (skin cancer) cells
and may be especially useful in deactivating the carcinogens in
cigarette smoke and chewing tobacco. Curcumin generates an
anticancer effect by inhibiting nuclear factor kappa B (NF-κB),
and also reduces the formation of glycation end products which
induce inflammation. Curcumin also mediates anticancer activity
by targeting many other enzymes/pathways, maintaining levels of
vitamins C and E, preventing peroxidation of lipid, and DNA
damage. Curcumin targets transformed cells without altering
primary astrocytes. It also promotes apoptosis, and shows a
synergistic effect in combination cisplatin and doxorubicin
drugs. An active constituent of turmeric suppresses
carcinogenesis in multiple human carcinomas, which include
ovarian cancers, stomach cancer, colon cancer, breast cancer,
head and neck cancer. Curcumin suppresses the carcinogenesis by
targeting diverse molecular targets of cellular division and
apoptosis. The beneficial effects of curcumin on various
transcription factors, oncogenes, and signalling proteins are
well known. It also targets various stages of carcinogenesis
from the initial stage to tumorigenesis, growth, invasion, and
metastasis. Animal studies involving rats and mice as well as in
vitro studies utilizing human cell lines have demonstrated
curcumin's ability to inhibit carcinogenesis at three stages:
Tumor promotion, angiogenesis, and tumor growth. In two studies
of colon and prostate cancer, curcumin inhibited cell
proliferation and tumor growth. The anticarcinogenic effects of
turmeric and curcumin are due to direct antioxidant and
free-radical scavenging effects and their ability to indirectly
increase glutathione levels, thereby aiding in hepatic
detoxification of mutagens and carcinogens and in inhibiting
nitrosamine formation. A number of laboratory studies on cancer
cells have shown that curcumin does have anticancer effects.
These studies have found that curcumin can significantly inhibit
the growth, development and movement of cancer throughout the
body. It seems to be able to kill cancer cells and prevent more
from growing. It has the best effects on breast cancer, bowel
cancer, stomach cancer and skin cancer cells. An American study
that combined curcumin with chemotherapy to treat bowel cancer
cells in a laboratory showed that the combined treatment killed
more cancer cells than the chemotherapy alone. Another American
study seemed to show that curcumin helped to stop the spread of
breast cancer cells to other parts of the body. Doctors think
that curcumin stays in the digestive system and is absorbed by
the cells in the bowel. Several studies have shown that curcumin
taken as capsules does get absorbed by the gut and is present in
the blood. One of the mechanisms by which it does this is
reducing the growth of new blood vessels in tumors (otherwise
known as angiogenesis) and can also directly contribute to the
death of cancerous cells. Scientists discovered that turmeric is
effective in killing cancer cells and also preventing their
growth. According to the American Cancer Society, tests indicate
that curcumin "interferes with several important molecular
pathways involved in cancer development, growth and spread" and
has boosted the effects of chemotherapy in animals. Curcumin has
the potential for treatment of cancers including colon, breast,
prostate, lung, skin and bowel. Curcumin shows a strong ability
to kill cancer cells as well as inhibit their growth, boost
antioxidant levels and balance the immune system. It seems to
work on improving mitochondrial function at a cellular level.
Even against drug-resistant strains of leukemia, curcumin caused
cell death of cancer cells. Curcumin has been shown to
substitute chemotherapy for colorectal cancers, and in
multidrug resistant cancers. The ability of curcumin to regulate
a variety of signaling pathways involved in cell growth,
apoptosis, invasion, metastasis, and angiogenesis in preclinical
studies elicited scientific interest in its potential as an
anticancer agent in tumor therapy. Curcumin is one of the most
powerful and promising chemopreventive and anticancer agents,
and epidemiological evidence demonstrates that people who
incorporate high doses of this spice in their diets have a lower
incidence of cancer. Curcumin's epigenetic modulation has been
studied by the US National Cancer Institute (NCI) and academic
investigators around the world. Because of low toxicity and
great efficacy in multiple in vitro and in vivo cancer models,
Curcumin was selected for further development, put through
extensive toxicology testing and has successively made it
through the first stages (Phase I) of clinical testing abroad
and is currently in clinical trials at several sites in the U.S.
A phase I clinical trial looked at giving curcumin to 25
patients with pre cancerous changes in different organs. This
study seemed to show that curcumin could stop the precancerous
changes becoming cancer. Numerous mechanisms have been described
for the anticancer activity of Curcumin. Curcumin inhibits the
NF-ŒB and STAT3 signaling pathways, which play key-roles in the
development and progression of cancer. It inhibits a highly
expressed transcription factor Sp-1 and its downstream genes,
including ephrin type-B receptor 2 precursor, HDAC4, calmodulin
and ADEM10 which serve as an important mechanism to prevent
metastasis. Curcumin enhances the expression of several
extracellular matrix components and inhibits the phosphorylation
of focal adhesion kinase (FAK) and CD24 expression, thus
prevents cancer formation, migration and invasion (Vallianou et
al. 2015; Shi et al. 2001; Zhou et al. 2013). In addition, the
potential mechanism of the anti-invasive effect of curcumin
includes downregulation of Akt, EGFR, cyclin D1, cMET and
upregulation of DNAJ/heat shock protein (HSP) 40 chaperone.
Recent studies revealed that ER stress and autophagy might
involve in apoptosis process. Mechanistically, autophagy
inhibition could increase curcumin induced apoptosis by inducing
ER stress (Vallianou et al. 2015). Further, the anticancer
effects induced by phytoconstituent curcumin in malignant cells
are mediated via the modulation of multiple signaling pathways
and its effectors. Curcumin induced anti-carcinogenic effects
includes down-regulation of the insulin-like growth factor
type-1 receptor (IGF-1R), EGFR/avian erythroblastosis oncogene
B1 (erbB1), erbB2/human epidermal growth factor receptor 2
(HER2), Wnt/b-catenin and sonic hedgehog/glioma associated
oncogene (SHH/GLIs), and their respective downstream signaling
effectors. Curcumin modulates intra-cellular signal transduction
elements such as p21, p27, inhibitor of growth family member 4
(ING4), cyclin D1, c- Myc, VEGF, ICAM-1, MMPS, uPA, COX-2,
CXCR-4, Bax, Bad, Bak, Noxa, p53, modulator of apoptosis,
caspases etc. resulting in reversal of cancer incidence,
progression and relapse (Jordan et al. 2016; Mimeault
and Batra 2011; Kasi et al. 2016). A2012 study
indicates that rates of colorectal cancer in India are among the
lowest in the developed world. Another study from 2016 shows
that Indian women are less likely to be diagnosed with breast
cancer, as well. Part of the reason for the lowered cancer rates
in India has been attributed to diet, with turmeric and curcumin
being major dietary contributors in that part of the world.
Combining curcumin with anticancer
drugs like gemcitabine in pancreatic cancer, docetaxel in breast
cancer, and imatinib in chronic myeloid leukemia may be safe and
well tolerated. A recent single-arm, phase II trial combining
three cycles of docetaxel/prednisone and curcumin (6 g/day) was
carried out in 26 patients with castration-resistant prostate
cancer. The level of prostate-specific antigen (PSA) was
decreased in most patients and was normalized in 36% of them,
and the co-administration of curcumin with drugs showed no
toxicity beyond adverse effects already related to docetaxel
monotherapy. Many registered phase I/II clinical trials designed
to investigate the effectiveness of curcumin alone or with
first-line treatment in patients with breast, prostate,
pancreatic, lung, or colorectal cancer are under way. Research
into preventing cancer: A phase I clinical trial looked at
giving curcumin to 25 patients with pre-cancerous changes in
different organs. This study showed how curcumin could stop the
precancerous changes becoming cancer. A number of laboratory
studies on cancer cells have shown that curcumin does have
anticancer effects. It kills cancer cells and prevent more from
growing. It has the best effects on breast cancer, bowel cancer,
stomach cancer and skin cancer cells. A study that
combined curcumin with chemotherapy to treat bowel cancer cells
in a laboratory showed that the combined treatment killed more
cancer cells than the chemotherapy alone. An American study
in mice showed that curcumin helped to stop the spread of breast
cancer cells to other parts of the body. Doctors think that
curcumin stays in the digestive system and is absorbed by the
cells in the bowel. Several studies have shown that curcumin
taken as capsules does get absorbed by the gut and is present in
the blood. A number of activities of curcumin, which are exerted
in a chemopreventive and a directly therapeutic manner, indicate
that it may be a potential anticancer remedy. Researchers at
M.D. Anderson Cancer Center in Houston, TX state that Curcumin
has “enormous” potential to prevent and treat cancer. Curcumin
was able to suppress tumor formation, growth, and even
metastasis according to their review. Currently, there are
clinical trials being conducted on the effects of Curcumin on
patients with bowel cancer. According to the American Cancer
Society, tests have shown that curcumin can kill cancer cells in
laboratory dishes, and also slow the growth of the surviving
cells. Furthermore, it has been found to reduce the development
of several forms of cancer in lab animals, while also shrinking
various animal tumors. A review -
Anticancer Potential
of Curcumin: Preclinical and Clinical Studies - in Anticancer Research
concluded that, "…it is quite apparent that curcumin has tremendous
potential for prevention and therapy of various cancers." Another study on
the role of curcumin in cancer therapy
found that, curcumin is a potent anti-inflammatory agent
with strong therapeutic potential against a variety of cancers. Curcumin has
been shown to suppress transformation, proliferation and metastasis of
tumors, and called for additional and larger controlled studies to
determine its full potential. Inhibition of proliferation
of tumor cells, induction of apoptosis (a mode of cell death), inhibition of
transformation of cells from normal to tumor, inhibition of invasion and
metastasis and suppression of inflammation have been linked with the
activity of curcumin. Resistance to chemo and radiotherapy is the major reason
for cancer relapse. This arises due to the presence of a
subpopulation of cancer cells, having self-renewal capabilities
called Cancer Stem Cells (CSCs). Studies have confirmed that
curcumin could inhibit the breast cancer stem cell population by
downregulating the expression of stem cell genes Oct4, Sox2 and
Nanog and also the Epithelial-Mesenchymal Transition (EMT) as
observed by the down-regulation of mRNA levels of Vimentin,
Fibronectin and β-catenin and up-regulation of mRNA levels of
E-cadherin (Hu et al., 2019). A combination of sub-optimal dose
of 5-FU and curcumin elicits synergistic antitumor potential in
murine models as evaluated by a reduction in the tumor-related
parameters. Mechanistically, curcumin down-regulates 5-FU
induced up-regulation of Thymidylate Synthase (TS), which is
responsible for 5-FU chemoresistance (Vinod et al., 2013;
Haritha et al., 2021). Another study reported that cervical
cancer cells can be sensitized by curcumin to paclitaxel-induced
apoptosis through down-regulation of NF-κB, Akt and Bcl2 (Bava
et al., 2011). The chemopreventive agent curcumin also act as a
potent radiosensitizer in human cervical tumor cells. Curcumin
pre-treatment increased reactive oxygen species production and
overactivation of the mitogen-activated protein kinase pathway
in HeLa and SiHa cells when treated with Ionising Radiation (Javvadi
et al., 2008). Mechanism of action of curcumin mainly
involves down-regulation of transcription factor NF-κB by
inhibition of Notch signalling, which is involved in cell
proliferation, apoptosis, maintenance of stem cell and their
renewal. This results in a reduction in expression of NF-κB
regulated genes, which includes Bcl-2, cyclin D1 and VEGF (O’riordan
et al., 2005). Curcumin is a strong inhibitor of Protein Kinase
C (PKC) and several oncogenes such as c-jun, c-fos, c-myc, NIK,
MAPKs, ERK, ELK, PI3K, Akt and CDKs. Curcumin also inhibits of
the Notch-1 downstream target Hes-1 in esophageal cancer cells.
Hes-1 is an important notch signalling target and mediator (Subramaniam
et al., 2012). The curcumin analog, 2-pyridylcyclohexanone has
also been shown to decreases basal STAT3 phosphorylation and
promotes apoptosis in esophageal cancer cell, ESCC cells (Wang
et al., 2018). Curcumin quenches free radicals, induces
antioxidant enzymes (catalase, superoxide dismutase, glutathione
peroxidase), and up-regulates antioxidative protein markers,
Nrf2 and HO-1 that led to the suppression of cellular oxidative
stress. In cancer cells, curcumin aggressively increases ROS
that results in DNA damage and subsequently cancer cell death (Ak
and Gülçin 2008). Curcumin was found to suppress inflammatory
cytokines such as IL-6, IL-8, granulocyte macrophage colony
stimulating factor and TNF-α as well as IKKβ kinase in the
saliva of HNSCC patients. Kim SG., et al., also suggested that
IKKβ kinase could be a plausible biomarker for the detection of
the effect of curcumin in head and neck cancer as curcumin
inhibited IKKβ kinase activity and this resulted in the reduced
expression of a number of cytokines (Kim et al., 2011).
Molecular docking studies further aids in identifying the role
of curcumin in numerous signalling cascades involved in
carcinogenesis and confirms the already suggested molecular
mechansims responsible for the chemopreventive efficacy of
curcumin. Using inverse molecular docking several proteins
associated with cell proliferation and tumor formation namely,
macrophage colony stimulating factor 1 receptor, aldo-keto
reductase family 1 member C3, amiloride-sensitive amine oxidase
and tyrosine-protein phosphatase non-receptor type 11 were
identified as potential targets of curcumin. Curcumin was
previously reported to inhibit the NFkB mediated activation of
genes linked to cell survival and proliferation (Divya and
Pillai 2006). Proteins such as MMP-2, NAD-dependent protein
deacetylase sirtuin-2, core histone macro-H2A.1, NAD-dependent
protein deacetylase sirtuin-1 and epidermal growth factor
receptor were also revealed to be targets of curcumin, the
binding of which regulates the activity of NF-kB (Furlan et al.,
2018). These results provide a mechanistic explanation for the
anticancer effects of curcumin. Targeting Phosphodiesterase 4
(PDE4) has been reported to be a potential therapeutic strategy
against inflammatory disorders (I Sakkas et al., 2017). Studies
suggest that curcumin may exhibit its anti-cancer property
through the inhibition of PDE2 and PDE4 (Abusnina et al., 2015).
Furlan et al. also gives evidences for the inhibitory effect of
curcumin on PDE4 (Furlan and Bren 2021).
Curcumin as an Anti-Inflammatory
| It has been observed
that chronic inflammation is responsible for several diseases,
such as tumor progression, autoimmunity, allergies, and
arthritic syndromes. Numerous researches revealed that curcumin
can decrease pro-inflammatory cytokines such as IFN-γ, TNF-α,
IL-1, and IL-8 by interfering with several signaling and
transcription factors such as NF-кB, JAKs/STATs, and MAPK
pathways (64). The anti-inflammatory activity of curcumin mainly
depends on its potentiality to inhibit NF-kβ activation.
Curcumin inhibits inflammation by downregulating cytokines,
IL-1, IL-8, and TNF-α. Curcumin blocks TNF-mediated NF-кB
activation in human myeloid ML-1a cells by suppressing activator
proteins. Curcumin also blocks NF-кB activation by hydrogen
peroxide and phorbol esters. IL-1β-mediated ICAM-1 and IL-8 gene
expression are also inhibited by curcumin, which finally leads
to the inhibition of NF-кB activation. JAK/STAT is an important
signaling pathway in maintaining inflammation in immune cells.
It transduces signal type 1 and 2 cytokine receptors in response
to pro-inflammatory cytokines. Curcumin inhibits JAK/STAT
pathway by blocking the phosphorylation of JAK-1 and -2 and
STAT-1 and -2 in IFN-γ, gangliosides, and LPS-activated
microglial cells. Curcumin has a distinct role in the
inflammatory MAPK pathway. Curcumin significantly lowers the
PGE2 (prostaglandin E2) level and the expression of TNF-α and
IL-6 by preventing phosphorylation and activation of p38 MAPK
functioning. Curcumin can suppress LPS-induced phosphorylation
of p38, JNK, and ERK1/2-mediated MAPKs pathways and subsequently
inhibit the ROS production by microglial cells (70). Kim et al.
validated that if immature DCs cells pre-treated with curcumin,
it blocked the LPS-induced maturation function of DCs by
preventing phosphorylation of p38-, JNK-, and ERK1-/2-mediated
MAPK signaling, which consequently checks the inflammation
occurrence .It is well
known that the human body is capable of self-healing after a
short-term inflammatory response, but long-term chronic
inflammation could lead to initiation of the cancer process.
Many studies have shown that inflammatory factors (including
interleukins, TNF-α, NF-ϰB) and the ROS production-induced
inflammation infiltrate the inflammatory microenvironment
leading to DNA damages and ultimately initiation of cancer. By
acting on several signaling pathways, especially the
WNT/β-catenin pathway, curcumin can have anticancer effect by
inhibiting chronic inflammation and oxidative stress. The
chronic inflammatory microenvironment of tumors could also be
targeted by curcumin.
Immunomodulatory Role of
Curcumin | Curcumin can inhibit the expansion of T
cells triggered by plant lectin concanavalin A (Con A),
according to a report on the role of the genus Curcuma and its
bioactive metabolites to control the immunological response.
Curcumin inhibits lymphoma B-cell proliferation by lowering the
potency of c-MYC, BCL-XL, and NF-κB. Curcumin has also been
demonstrated to suppress the production of ROS in macrophages.
Curcumin also stimulates NK cell apoptosis by modulating the
NF-κB pathway and inhibiting BCL-XL and Cyclin D. Curcumin
inhibits IL-1 and IL-6 inflammatory cytokines such as from
LPS-stimulated dendritic cells and suppresses the expressions of
CD80, CD86, and MHC class II by dendritic cells. Curcumin also
causes reduced LPS-induced MAPK activation and NF-κB p65
translocation in dendritic cells (Nair et al., 2017) along with
impaired activation of Th1 responses. Curcumin significantly
suppressed the formation of IL-6, IL-8, TNF-α, and MCP-1 from
higher glucose-cultured monocytes, according to Jain et al.
(2009). Curcumin decreased NOS activity and macrophages’ ability
to secrete nitric oxide (NO). In the management of immune modulation,
curcumin treatment can promote the activation of immune
component cells, including reduce excessive activation of
inflammation and allergy, and enhance endogenic immune activity
to fight foreign pathogens or cancer cells. Remarkably, curcumin
can suppress intracellular NF-κB, MAPKs, JAKs/STATs, β-catenin,
and the Notch-1 pathway by regulating the expression and
secretion of pro-inflammatory cytokines, such as IL-1β, TNF-α,
IL-2, IL-6, IL-10, which mediate inflammatory pathways. In the
managements of clinical treatment, curcumin can also be applied
to autoimmune diseases therapies, such as lupus erythematosus,
rheumatoid arthritis, ankylosing spondylitis, psoriasis, etc.
Curcumin treatment can restore cellular immune-reactive T cells
and assist the body to fight endogenous cancer cells and
exogenous pathogens. In vitro and in vivo data showed curcumin
can hinder cancer cell proliferation or cause cancer cell
apoptosis. Curcumin as an immunomodulator interacts not
just with various cellular components, such as DCs, macrophages,
natural killer cells, and both B and T lymphocytes, but also
with modulatory molecules involved in the processes of
inflammation and cell proliferation with their downstream
signaling. In recent times, curcumin has gained the potential
therapeutic interest to cure neoplastic disease, because of its
significance as an anti-inflammatory and anti-proliferative
substance. The anti-cancer properties of curcumin also modulate
several other signaling pathways involved in mutagenesis,
oncogene expression, cell cycle regulation, apoptosis,
angiogenesis, and metastasis. The effectiveness of curcumin has
been proven in the restoration of CD4+ and CD8+ cells in the TME
and in directing Th2 cytokine bias towards Th1-type response
again. It increases Th1-type immune responses and upregulates
IFN-γ mRNA expression. Curcumin effectively reduces Treg cell
population and levels of IL-10 and TGFβ. It also can reduce the
expression of CTLA4 and FOXP3 both at protein and mRNA levels
(55). Interestingly, curcumin has the potentiality to encounter
all “six hallmarks” of cancer cells and checks tumor outgrowth
in the host. Hence, it is considered very interesting to
envision the role of curcumin concerning cancer immunotherapies
as an immunomodulator.
Effects of Curcumin on Immune
Cells | Curcumin has the potentiality to
modulate the proliferation and activation of T cells. Depending
on the dose, it can both suppress and induce the proliferation
of T cells. Several studies reported that curcumin downregulates
the proliferation of T cells induced by concanavalin A (Con A),
phytohemagglutinin (PHA), and phorbol-12-myristate-13-acetate
(PMA). Tomita et al. reported that curcumin can suppress the
proliferation of HTLV-1-infected T cells and primary ATL cells
through cell cycle arrest and induction of apoptosis. Research
carried out by Hussain et al. stated that in T cell acute
lymphoblastic leukemia, curcumin blocks constitutively activated
targets of PI3-kinase (AKT, FOXO, and GSK3) in T cells, which
lead to the inhibition of proliferation and induction of
caspase-dependent apoptosis. On B cell: Curcumin prohibits the
proliferation of B-cell lymphoma cells via downregulation of
c-MYC, BCL-XL, and NFκB activities. It also blocks Epstein–Barr
virus (EBV)-induced immortalization of B-cells. On macrophage:
Curcumin modulates macrophage activities, prevents generation of
ROS in macrophages, and stimulates enhanced phagocytosis of
peritoneal macrophages in mice. On Natural Killer cell: Curcumin
works against natural killer T cell lymphoma cell lines, where
it induces apoptosis by controlling the NFκB pathway and
suppression of BCLXL, Cyclin D1, etc.. On DC: Curcumin can
reduce the expression of CD80, CD86, and class-II antigens by
DCs. Curcumin suppresses the release of inflammatory cytokines
like IL-1β, IL-6, and TNF-α from LPS-stimulated DCs. Curcumin
also modulates phosphorylation of MAPK and nuclear translocation
of NFκB in DCs.
Curcumin as an Anti-Proliferative and
Anti-Metastatic Substance | Curcumin acts upon numerous
cell proliferation signaling pathways that are intensely
associated with cancer progression. Curcumin inhibits NF-кB
signaling by suppressing IкB kinase activity. Curcumin
suppresses the other proliferation signaling pathways, such as
PI3K, AKT, mTOR, AP1 (JUN and FOS), JNK, JAK/STAT, PKC, CMYC,
MAPK, ELK, CDKs, iNOS, and Wnt/β-catenin, which confirmed its
vital role in the prevention of cancer progression. Cyclin D1,
the proto-oncogene that is highly expressed in several types of
cancer and acts in cell cycle progression and proliferation, is
also suppressed by curcumin. Along with this, curcumin also
inhibits excessive TGFβ receptor signaling and EGF- and
EGFR-mediated signaling pathway and remarkably controls
epithelial-to-mesenchymal transition, metastasis, and tumor
progression, respectively. A significant activity of the
telomerase enzyme has been observed in cancer cells, which
prevents telomere shortening and stimulates continuous cell
proliferation signaling. Curcumin prevents human telomerase
(hTERT) activities and reduces hTERT-mRNA expression that led to
telomere shortening. By targeting telomerase activities,
controlling replicative cell senescence and mortality, curcumin
ultimately controls the uncontrolled cell proliferation of
cancer cells. Numerous studies have reported the incredible
potentiality of curcumin to inhibit cell migration, invasion,
and colony formation in vitro and decrease tumor growth and
metastasis in vivo. Curcumin downgrades the expression of matrix
metalloprotease, CCRX4, COX2, ELAM1, and ECAM1, which are
essential for metastasis. Besides, curcumin also hampers the
functioning of SLUG, SNAIL, FAK, TWIST, and other essential
transcription factors that play a crucial role in the metastasis
process.
Curcumin as an Apoptotic and Anti-Angiogenic
Substance
Curcumin has been suggested as an enhancer of apoptosis in
cancer cells. It has the ability to modulate a wide range of
signalling pathways involved in apoptosis resistance in cancer
cells. Curcumin also triggers programmed cell death in colon
cancerous cells and inhibits micro-inflammation in the
gastrointestinal system linked to inflammatory bowel illnesses,
according to laboratory research (Nita, 2003). Okanlawon et al.
(2020) determine the influence of the inclusion of powdered C.
longa on carcass yield and intestinal increase in rabbit
production. Farombi et al. (2007) explored the combined effects
of curcumin and kolaviron (a bioflavonoid extracted from
Garcinia kola seeds) on DBP-induced testicular injury in rats.
Curcumin treatment of mice infected with human prostate cancer
cells resulted in a lowered microvessel density, cell
proliferation, an improvement in apoptosis. Endothelial cells
derived from bovine aorta exposed to curcumin (5–15 μM) under
normoxic (oxygen tensions within 10–21%) or hypoxic (oxygen
tensions within 1–5%) conditions were reported to increase heme
oxygenase activity and resistance to oxidative stress.
Consumption of alcohol sensitizes the pancreas to give an
inflammatory response through NF-κB activation via protein
kinase C epsilon. One pilot study concluded that an oral dosage
of 500 mg of curcumin with 5 mg of piperine could restore lipid
peroxidation in patients suffering from tropical pancreatitis (Durgaprasad
et al., 2005).A common property for most cancer cells is
some mutations in tumor suppressor genes, especially p53 and
PTEN. Mutations in these genes lead to them escaping cell death
and also resistance to therapy. Curcumin has the ability to
reverse the activities of both p53 and PTEN. Inhibition of
mir-21 has a key role in the activation of PTEN, leading to
inhibition of the PI3K/AKT pathway. Suppression of AKT following
treatment with curcumin can cause degradation of MDM2, which
leads to activation of p53. Furthermore, suppression of AKT can
reduce the expression of anti-apoptotic genes such as COX-2,
NF-κB and Bcl-2. Downregulation of these genes by curcumin has
shown its potential to sensitize a wide range of cancer cells to
chemotherapy and radiotherapy. In addition to the regulation of
a wide range of genes in cancer cells, curcumin has been shown
to modulate the tumor microenvironment in favor of tumor
suppression. Inhibition of some immunosuppressive cells'
cytokines such as IL-10 and TGF-β leads to more infiltration and
proliferation of NK cells and CTLs as well as inhibition of
tumor-promoting cells including TAMs, CAFs and Tregs. These
immunoregulatory effects of curcumin lead to the release of dead
signals such as FasL and TNF-α for cancers. Upregulation of Fas,
TNFR and TRAIL because of elevated ROS production by cancer
cells can facilitate apoptosis pathways following treatment with
curcumin. Curcumin blocks the
phosphorylation of another tumor suppressor protein, RB
(Retinoblastoma), which plays a significant role in the cell
cycle process. Curcumin induces both TP53-dependent and
-independent apoptosis of cancer cells by upregulating
pro-apoptotic molecules such as BAX, BIM, and PUMA and by
downregulating anti-apoptotic molecules like BCL2, BCL-XL, and
Survivin. Consequently, the caspase activity gets enhanced and
proceeds to apoptosis. Besides, curcumin stimulates lysosomal
proteases, phosphatases, and lipase activities, which induce
autophagy-mediated cell death. Blocking the angiogenesis process
is a vital step to control tumor outgrowth. Curcumin suppresses
VEGF receptor (VEGFR1 and VEGFR2) expression, blocks
VEGF/VEGFR-mediated signaling pathway, and downregulates
angiopoietin expression to confine angiogenesis. In vitro and in
vivo studies have indicated that curcumin prevents
carcinogenesis by affecting two primary processes: angiogenesis
and tumor growth. Turmeric and curcuminoids influence tumor
angiogenesis through multiple, interdependent processes: i)
action at the level of transcription factors associated with
inflammatory processes and early growth response protein which
reduces the expression of IL8 in pancreatic and head and neck
cancer cell lines and prevents the induction of VEGF synthesis;
ii) inhibition of angiogenesis mediated by NO (nitric oxide) and
iii) inhibition of COX2 and 5LOX; iv) action at the level of
angiogenic factors: VEGF, the primary factor for migration,
sprouting, survival and proliferation during angiogenesis, and
basic fibroblast growth factor. Because of its anti-apoptotic
and antiproliferative efficacy, its ability to interfere with
several tumor progression associated signaling pathways, and to
modulate tumor-associated miRNA expression, curcumin is regarded
as antitumorigenic. In addition, curcumin prevents formation of
breast and prostate metastases in vivo. The review by
Willenbacher et al. in this issue summarizes some papers that
have been published in the field of curcumin’s antitumorigenic
effects. Curcumin is also potent against cancer types that are
difficult to treat, like melanoma or glioblastoma, as
demonstrated by the work of Maiti et al. in this issue. An
American phase 2 study reported in 2008. 25 patients had
curcumin treatment and 21 had tumors that could be measured. In
2 patients their tumors shrank or remained stable. In some
patients their levels of particular immune system chemicals that
destroy cancer cells went up. Curcumin also has been studied
with regards to the core inflammatory gene signal,
NF-kappaB, resulting in a beneficial domino effect throughout the
body. One benefit of this domino effect is a direct reduction in
the risk of cancer from overweight-induced inflammation.
Curcumin has been found to induce cell-cycle arrest and
apoptosis by regulating a variety of cell-signaling pathways (3,
41-45). For example, the inhibition of cell proliferation by
curcumin has been associated with the Nrf2-dependent
downregulation of DNA repair-specific flap endonuclease 1 (Fen1)
in breast cancer cells in culture. Curcumin has been shown to
induce p53-dependent or -independent apoptosis depending on the
cancer cell type. In a panel of cancer cell lines,
p53-independent apoptosis induced by curcumin was mediated by
the rapid increase of ROS and the activation of MAPK and c-jun
kinase (JNK) signaling cascades. Inhibition of NF-κB signaling
by curcumin also suppresses proliferation and induces apoptosis
in cancer cells.
Effect of Curcumin on
Chemoresitance | Curcumin has a proven ability to
counter chemoresistance in cancer cells. The ability of curcumin
to modulate the regulatory networks governing the balance of
cell survival and induction of cell death is well established.
Curcumin has been demonstrated to amend the expression of
molecules central to chemoresistance including members of the
ABC drug efflux transporter family. Curcumin modulates the
cancer metabolism and bio-physiological composition of the
extracellular milieu culminating in the induction of cell death
and retardation in disease progression. Metabolic alterations
and suppression of receptor-mediated signaling were suggested to
provide chemosensitization of cancer cells by curcumin. The
previous investigation on hepatic carcinoma cells demonstrated
that curcumin can thwart lactate-induced chemoresistance.
Interestingly, curcumin can be exploited to provide health
benefits in diabetes mellitus owing to its antioxidant and
anti-inflammatory capabilities. In renal tubular epithelial
cells, curcumin was also shown to obviate high glucose-induced
epithelial-to-mesenchymal transition. High glucose conditions
can aggravate the invasion and migration, while curcumin can
impede the metastatic events in a variety of malignancies.
Antineoplastic potential, metabolic modulatory ability, and
chemosensitizing property along with safety investigations
provide an edge to curcumin over other phytochemicals. Curcumin
has the ability to avert high glucose-induced chemoresistance in
cancer cells. Various aspects of the underlying mechanism were
also explored. Curcumin mediated the amputation of
chemoresistance by repressing the hyperglycolytic behavior of
malignant cells via modulated expression of metabolic enzymes
(HKII, PFK1, GAPDH, PKM2, LDH, SDH, IDH, and FASN), transporters
(GLUT-1, MCT-1, and MCT-4), and their regulators. Along altered
constitution of extracellular milieu, these molecular changes
culminated into improved drug accumulation, chromatin
condensation, and induction of cell death. Molecular alterations
in the expression level of transcription factors (p53, HIF-1α,
MYC), drug efflux pumps (MDR-1), and their regulators (HCAR-1,
mTOR, and STAT3) can be suggested as the underlying molecular
mechanism. This investigation contributed to the understanding
of the anticancer ability of curcumin through the prevention of
chemoresistance in hyperglycemic conditions along with
underlying mechanisms. The demonstrated potential of curcumin
against high glucose-induced chemoresistance will have
implementations in clinical management of malignancies in
diabetic patients.
Curcumin as an Anti-Tumor Substance | The anti-tumour
activity of curcumin is mediated via anti-inflammatory,
apoptosis-inducing, anti-oxidative and anti-angiogenic
activities. In colon cancers, the anti-tumour activity of
curcumin was mediated via inhibition of COX-2. P53
(apoptosis-inducing in stressful situations) has been shown to
have a varied response to curcumin administration;
overexpression in human hepatoblastoma, human breast cancer
cells and human basal cell carcinoma and downregulation in
colorectal carcinoma reveal that it may be tissue-specific. Its
anti-angiogenic effect is by inhibition of angiogenic factors
like fibroblast growth factor (FGF), ligands of VEGF and
angiopoietin 1 and 2 and regulation of cell adhesion molecules
like endothelial adhesion molecule-1 (ELAM-1), intracellular
adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1
(VCAM-1) and cell surface proteins that are involved in tumour
metastasis. Implementation of
curcumin reduces cell mutations caused by exposure to
carcinogens and induces the body's anti-tumor responses.
Curcumin can promote M1-like tumor microglia activation and
increase recruited natural killer cell to cause tumor
decimation. Curcumin can inhibit the cancer cell proliferation
by inhibit NF-κB, COX-2, CD-31, VEGF, and IL-8, matrix
metalloproteinase (MMP)− 9. Turmeric extract or curcumin
can inhibit the proliferation of cancer cells such as head and
neck cancers, lung cancer, digestive system cancer,
urinary system cancer, and reproductive system cancer in vitro
and in vivo. The oral submucosal fibrosis, which is highly
associated with oral cancer, is a chronic latent disease that
leads to the hardening of the oral mucosa and deep tissues, and
extends to the wall of the throat or esophagus, gradually
causing difficult or loss of eating, swallowing, and
pronunciation. In clinical management of oral mucosal fibrosis,
curcumin is evident to treatment can prevent, reduce, and
improve oral mucosal fibrosis. Curcumin treatment can increase
the levels of vitamins C and E in salivary and serum to enhance
organism’s antioxidative values, and decrease the malonaldehyde,
8-hydroxydeoxyguanosine (8-OHdG) to decrease oxidative stress.
In clinical application, curcumin is used as an adjuvant or
supplement for chemotherapy or nuclear therapy in clinical tumor
treatments to reduce postoperative adverse reactions, which
still requires clinical verification. Curcumin has
multiple potentials due to its numerous antineoplastic
mechanisms for cancer therapy. Curcumin, a chemo-sensitizing
agent, also enhances the efficacy of several chemotherapeutic
agents. According to the study by Chen’s group, among four
anticancer chemotherapeutic factors (erlotinib, sorafenib,
sunitinib, and doxorubicin), sunitinib combined with curcumin at
a molar ratio of 0.46 achieved the most potent synergistic
effect in vitro and has been selected to be studied in an animal
model. The anti-tumor effects of curcumin or turmeric extract in
combination with bevacizumab in HT29 colon tumor-bearing mice
have been examined by Yue’s group. When curcumin is combined
with bevacizumab therapy, it suppressed tumor growth
significantly with no physical side effects. This highly
indicates the therapeutic promise of adjuvant application of
curcumin for treating cancer, particularly combined with several
mAbs. A clinical trial has been executed by Basak et al. with
oral cancer patients where APG-157, a botanical drug containing
multiple polyphenols, including curcumin has been administrated
orally. According to the study, APG-157 was absorbed well and
significant trace of curcumin has been found in the blood and in
tumor tissues. This trial reported the downregulation of
inflammatory markers and Bacteroides species in the saliva and
upregulation of the immune T cells in the tumor tissue.
Additionally, it reduced inflammation and attracted cytotoxic T
cells to the tumor site, signifying its potential usage in
combination with immunotherapy drugs. Curcumin alone induced a 49–55% reduction in mean ovarian
cancer tumor growth compared with control animals, while the
combination of curcumin with docetaxel resulted in a 77%
reduction in mean tumor growth compared with the controls. In an
animal study, the administration of curcumin decreased the
number of lung tumor nodules and inhibited lung metastasis of
melanoma. Therefore, it is possible to use curcumin in order to
arrest the metastatic growth of tumor cells.
A
study
conducted in 2014 revealed that curcumin was able to
obstruct tumor growth and metastasis in several animals’ organs
including the stomach, colon, and liver.
Effect of
Curcumin on Lung Cancer |
Cancer stem cell-based treatments with curcumin could be proven
as potential processes and targets for tackling lung cancer (Ye
et al., 2012). Curcumin also seems to promote tumor progression,
reducing the efficiency of docetaxel in lung cancer patients.
The therapeutic effect of curcumin has also been exploited in
lung cancer. A mechanistic approach was used to study curcumin’s
anti-cancer potential, by targeting JAK2/STAT3 and NF-κB
signaling pathways, in the A549 lung cancer cell lin. In
addition, curcumin, via PI3K/Akt signaling suppression and
microRNA-192-5p up-regulation induced apoptosis in non-small
cell lung cancer cells with inhibition of cell proliferation.
In vivo curcumin lessens the migratory and invasive
capabilities of A549 cells and inhibited adiponectin expression
thought to be mediated through the NF-κB/MMP pathways and has
been proposed as an adjuvant in lung malignancy (Tsai et al.,
2015). Curcumin against human non-small cell lung cancer cell line
A549, which showed 50% cell viability at a high dose of 10,000 U
of interferon (IFN)-alpha (IFNα), was investigated to understand
the resistivity of these cells against such a higher
concentration of IFNα[64]. On treatment with one-tenth of the
IC50 value, the A549 cells showed an increase in p50 (NF-κB1)
and p65 (RelA) subunits of NF-κB with respect to time, in
addition to an increase in Cox-2 expression. On pretreatment
with curcumin, a dose-dependent decrease in these subunits was
noticed in Western Blot Analysis and a decrease in Cox-2
expression was also noted. Thus, curcumin showed a remarkable
decrease in NF-κB and Cox-2 activity in a dose-dependent manner
with a maximum dose of 50 μM in IFNα resistant A549 cell lines
and it increased the vulnerability of cells towards the
cytotoxic activity of IFNα. Animal
study revealed that curcumin administration reduced
ultra-histoarchitecture and histoarchitecture abnormalities
against benzo[a]pyrene induced lung carcinogenesis in mice (Wang
et al. 2016c). In in vitro studies, curcumin treatment is
reported to induce miR-98 and suppressed MMP-2 and MMP-9 which
leads to inhibition of lung cancer in A549 cell line (Liu et al.
2017). Curcumin downregulated the expression of hTERT, induced
cytotoxicity and attenuated proliferation in A549 cell line, and
suggested as effective target for lung cancer therapy
(Sadeghzadeh et al. 2017). Curcumin treatment reduced
CD133-positive cells, reduced the formation tumorsphere,
downregulated the expression of lung cancer stem cells markers
like Oct4, aldehyde dehydrogenase isoform 1A1, CD133, CD44 and
Nanog alongside induced apoptosis and inhibited proliferation of
lung cancer cells. In addition, it reduced lung cancer via
inhibition of sonic hedgehog and Wnt/b-catenin signaling
pathways (Zhu et al. 2017). Curcumin treatment inhibits
hepatocyte growth factor induced epithelial-mesenchymal
transition and angiogenesis by inhibiting PI3K/Akt/mTOR signal
transduction regulated by c-Met in human lung cancer cell line
A549 (Jiao et al. 2016). Recent evidence suggest that curcumin
treatment effectively prevented lung cancer metastasis and
growth by downregulating microRNA (miR)-let 7c and miR-101 mediated expression of enhancer of
zeste homolog 2 along with downregulation of Notch1 expression
in human lung cancer cell lines (A549 and NCI-H520) (Wu et al.
2016). Curcumin inhibited
IL-6-induced proliferation, migration, and invasiveness of human
small cell lung cancer (SCLC) cells by reducing JAK/STAT3
phosphorylation (i.e., activation) and downstream genes coding
for cyclin B1, survivin, Bcl-XL, MMPs, intercellular adhesion
molecule 1 (ICAM-1), and vascular endothelial growth factor
(VEGF). The therapeutic efficiency of curcumin in lung cancer is
exhibited by the suppression of COX-2, EGFR, NF-κB, and
PI3K/Akt signaling pathway. An interesting study by Jeeyun Lee
et al. investigated if interferon (IFN)-α stimulation activates
an NF-κB in lung cancer cells, and if curcumin annuls IFN-α
dependent NF-κB activation and subsequently NF-κB-regulated
gene's (cyclooxygenase-2's) expression. They reported that the
aforementioned hypothesis was correct in the case of A549 lung
cancer cells and curcumin effectively down-regulate COX-2
expression through IFN-α-dependent activation of NF-κB. G
Radhakrishna Pillai et al. reported that curcumin IC50 of 50 μM
is required to induce in vitro apoptosis in A549 cells [50].
Lichuan Wu et al. highlighted the fact that curcumin could
inhibit cell proliferation, colony formation, and tumorspheres
in lung cancer cell line NCI-H460. The underlying mechanisms of
curcumin-induced tumorspheres suppression are mainly due to the
inhibition of the JAK2/STAT3 signaling pathway. Furong Liu et
al. showed that curcumin exerts a cytotoxic effect on NSCLC A549
cells by inhibiting the PI3K/Akt/mTOR pathway to promote
apoptosis and autophagy. It indicates that PI3K/Akt/mTOR signal
transduction pathway is a key pathway involved in the role of
curcumin in lung cancer. One of the studies showed the effect of
curcumin on erlotinib-resistant non-small cell lung cancer
(NSCLC) cells. The combination of erlotinib and curcumin
reduced tumor growth remarkably in vivo in erlotinib-resistant
NSCLC cells. Ping Chen et al. provided the evidence that
gefitinib-resistant NSCLC cells growth could be inhibited by
downregulating Sp1/EGFR activity and the receptor tyrosine
kinase pathways with the use of curcumin and gefitinib together.
They also validated that curcumin could be utilized, in the
treatment of NSCLC with wild-type KRAS and EGFR mutation, as a
sensitizer of EGFR-tyrosine kinase inhibitors (EGFR-TKIs).
Effect of Curcumin on Breast Cancer | Curcumin
against resistant breast cancer have resulted in promising
results. The maximum
tolerable dose of curcumin was found to be 8 g/day.
During the pathogenesis of cancer,
multiple signaling pathways are involved and curcumin represents
a potential candidate for the regulation of these signaling
pathways. Among these, pro-inflammatory transcription factor
(NF-κB) is involved in breast cancer cell proliferation.
Curcumin down-regulates the NF-κB signaling pathway, thus,
affecting the cell proliferation and invasion contributing to
breast cancer treatment. In another breast cancer model,
curcumin induced autophagy through down-regulation of Akt
protein, posing a significant management strategy for breast
cancer. These findings suggest the therapeutic potential of
curcumin following multiple signaling pathways.
Adriamycin resistant MCF-7ADR and Tumor Necrosis Factor
resistant BT-20TNF breast cancer cell lines showed 15% (± 6%)
and 8% cell viability respectively against curcumin at a dose of
1 μg/ml (2.7 μM). The same study claimed that curcumin
exhibited the growth inhibitory effect on estrogen-dependent
MCF-7 and T-47D as well as estrogen-independent SK-BR3 cell
lines at lower concentrations, and arrested the majority of
cells in the G2/M phase and inhibition of ornithine
decarboxylase (ODC) activity. A comparative study of the effect
of curcumin on human mammary epithelial (MCF-10A) and MDR breast
carcinoma (MCF-7/TH) cell lines reported that the IC50 value of
curcumin against MCF-10A was 3.5 times higher than that of
MCF-7/TH although cytometric analysis showed equal accumulation
of curcumin in both cell lines and it is well complemented with
the apoptosis studies where 40 µM (24 hr) concentration of
curcumin led 1.8% of MCF-10A cells into apoptosis while 46.6% of
MDR, MCF-7/TH went into apoptosis under similar conditions,
which in terms of considering the collateral damages is a
significant observation. In an investigation undertaken by
Meiyanto et al., doxorubicin-resistant breast cancer cell lines
MCF-7/Dox cells with over-expression of HER2 were tested against
doxorubicin (IC50 = 7) and curcumin (IC50 = 80 ± 2.39)
separately and in combination. The MTT Assay showed that
curcumin at half of its IC50 concentration in combination with
doxorubicin at half of its IC50 concentration, decreased the
percentage cell viability of MCF-7/Dox cell lines by almost 80%,
and this synergistic action of combinatorial treatment-induced
cell death, evident through the accumulation of more cells in
sub-G1 and G1 phase as compared to the percentage of cells when
they were treated separately by doxorubicin and curcumin.
Efficacy of curcumin against resistant breast cancer cell lines
was demonstrated through SRB assay on MCF-7,
antiestrogen-resistant MCF-7/LCC2 and MCF-7/LCC9 cell lines,
which revealed IC50 values of curcumin to be 9.7, 12.2 µM and
11.34 µM respectively against these cancer cell lines and colony
formation for each cell line was suppressed by curcumin at a
concentration of 30 µM. These activities of curcumin were
attributed to lowering of anti-apoptotic expressions and
inhibition of NF-κB and Akt/mTOR pathway. The photosensitization
of cancer cells by curcumin towards photodynamic therapy (PDT)
has been covered by Muniyandi et al. and apoptosis is the mode
of action in majority of the works cited in the review. The
adriamycin resistant breast cancer cell line MCF-7/ADR was found
to be equally affected as MCF-7 cells (Cell viability 50%) on 45
minutes preincubation with curcumin (7.5 μM) followed by
irradiation with blue light (450 nm, 100 mW/cm2) for 5 min and
subsequent 24 h incubation. Clinical trial study
recommended that, administration of curcumin (6 g/day for seven
consecutive days in every 3 weeks) in combination with docetaxel
to be safe, effective and well tolerated for advanced and
metastatic breast cancer (Bayet-Robert et al. 2010). In vitro
models revealed that curcumin treatment is known to induce
cytotoxicity through apoptosis induction and inhibit the
viability of MCF-7 cells via caspase-3 and 9 activations. It
reduced the expression of miR-21 by upregulating the PTEN/Akt
signaling in breast cancer cells (Wang et al. 2017).
Experimental evidence suggested that curcumin administration
downregulate the expression of estrogen receptor-alfa (ER-a) and
tumor suppressor protein exerting antiproliferative effects in
T-47D human breast cancer cells (Hallman et al. 2017). Besides,
curcumin treatment reduced hypermethylation of glutathione
S-transferase (GST) pi 1 (Kumar, Sharma, and Rathi 2017) and
deleted in liver cancer 1 (DLC1) (Liu et al. 2017),
downregulated the Sp1 and DNA methyltransferase 1 expressions,
resulting in inhibition of proliferation of human breast cancer
cells (Liu et al. 2017). A recent study revealed that curcumin
treatment downregulated the expression of Fibronectin, Twist1
Vimentin, AXL, Slug, b-catenin, N-cadherin and E-cadherin
thereby inhibited the migration and invasion of cancer in breast
cancer cell lines (Gallardo and Calaf 2016). Curcumin inhibits
NF-jB signaling resulting in inhibition of cell growth and
invasion in MDA-MB-231 human breast cancer cell line (Yodkeeree
et al. 2010). Further, curcumin arrested the cell cycle at the
late S and G2M phase alongside induced ROS mediated apoptosis,
accumulated p16/Rb and P53/p21 in breast cancer cells (Calaf et
al. 2011; Wang et al. 2016d). In breast cancer cells, curcumin
prevented EMT-associated morphological changes induced by
lipopolysaccharide (LPS) while upregulating E-cadherin and
downregulating vimentin. It was further shown that curcumin
inhibited NF-κB/Snail signaling involved in LPS-induced EMT. In
another study, curcumin increased the expression of the small
non-coding RNA miR181b, which then downregulated proinflammatory
cytokines, CXCL1 and CXCL2, as well as MMPs, thereby reducing
the metastatic potential of breast cancer cells. Compelling
evidence has demonstrated the benefits of curcumin combination
therapy as compared to monotherapy in breast cancer. An in vitro
investigation reveals that the co-administration of curcumin and
4-hydroxytamoxifen (4-OHT), a metabolite of tamoxifen, could
restore the sensitivity of 4-OHT of HR-positive MCF-7 cells
through the downregulation of cyclin D1 and upregulation of p21.
Compared to either curcumin or 4-OHT alone, combined treatment
also remarkably activated pro-apoptotic protein Bcl-xL and
suppressed the Bcl-2 proteins, thereby further enhancing the
apoptotic activities. The phenomenon was reversed with the
combined treatment of curcumin and 4-OHT in MDA-MB-231 cells,
mediating cell death and preventing the metastatic behavior of
breast cancer cells, respectively. Co-treatment of curcumin (10
μg/mL) and trastuzumab (10 μg/mL) significantly reduced cell
proliferation and induced G2/M arrest in HER2-overexpressed
BT-474 and SK-BR-3-hr (a herceptin resistant strain from
SK-BR-3) breast cancer cells, compared to trastuzumab alone.
Further in vivo study revealed that BT-474 xenograft mice models
had the smallest tumor volume after 4 weeks of curcumin (45
mg/kg) and trastuzumab (4 mg/kg) co-treatment. Curcumin also
serves as a potential adjuvant with other chemotherapeutic
agents in augmenting anticancer effects. The combined treatment
of curcumin and paclitaxel significantly suppressed the
paclitaxel-mediated NF-κB expression and its regulatory genes
COX-2, matrix metallopeptidase 9 (MMP-9), VEGF, and
intercellular adhesion molecule 1 (ICAM-1), thus promoting the
anti-proliferative and anti-metastatic behavior in breast cancer
cells. Interestingly, further experiments proved that curcumin
and paclitaxel curbed the metastasis of MDA-MB-435 breast cancer
cells to lung tissues in xenograft mice models. More
importantly, this combination of curcumin (ranging from 25–225
mg/kg) and paclitaxel (5 mg/kg) was found to be safe and induced
no toxicity effects in mice models. Hindered by drug efflux and
chemoresistance, doxorubicin was explored in combination with
curcumin in breast cancer treatment. This has successfully
augmented the cytotoxicity effect on breast cancer cells Another
study illustrated that curcumin inhibited the
doxorubicin-induced EMT via the suppression of Akt, β-catenin
and glycogen synthase kinase 3 β (GSK3β) protein expression,
emphasizing the importance of the combined treatment of curcumin
and doxorubicin in inhibiting the metastasis of breast cancer
cells. Apart from the combination with chemotherapeutic agents,
the combined treatment of curcumin with other natural compounds
has also been investigated in breast cancer. Flow cytometry cell
death analysis showed that the co-treatment of curcumin (5 μM)
and berberine (25 μM) synergistically exerted apoptosis and
autophagy cell death to MDA-MB-231 and MCF7 breast cancer cells.
Moreover, curcumin (1.5 μM) sensitized the AU565 breast cancer
cells treated with quercetin (4 μM) and optiberry (2 μg/mL) to
decrease lapatinib-mediated HER2 overexpression via the
downregulation of HER2/Akt signalling pathways. Another study
reported the benefits of curcumin (200 mg/kg) and
epigallocatechin gallate (EGCG) (25 mg/kg) in lowering the tumor
burden of xenograft models via the reduction in phosphorylated
Akt, EGFR and vascular endothelial growth factor receptor-1
(VEGFR-1) expression, highlighting the enhanced anticancer
potential of this treatment regimen. Curcumin is believed to
show its impact on cell growth and invasion of breast cancer
partially through the down-regulation of NF-κB signaling
pathways. Curcumin induces p53-dependent apoptosis and also
causes cell cycle arrest in MCF-7 breast cancer cells. In
curcumin-treated MCF-7 cells, proapoptotic protein B-cell
lymphoma-2 (Bcl-2)-associated X protein (BAX) was found in a
high concentration and it indicates curcumin's p53-dependent as
well as p53-independent antiproliferative effects. Xiao-Dong Sun
et al. identified that curcumin could inhibit the
phosphorylation of extracellular regulated protein kinase
(ERK1/2) in MDA-MB-231 cells. ERK1/2 is a major signaling
molecule in the downstream pathway of EGFR. This is how curcumin
inhibits cell proliferation and induces cell apoptosis, by
inhibiting the EGFR pathway in vitro in MDA-MB-231 cells. Yunus
Akkoç et al. reported that in metastatic MCF-7 breast cancer
cells, overexpression of B-cell lymphoma-2 (Bcl-2) is a
constraining factor for curcumin-induced apoptosis. The
overexpression of Bcl-2 blocks curcumin-induced autophagy
through its inhibitory interaction with Beclin-1 in MCF-7 cells.
They found that pre-treatment with LY294002, a PI3K inhibitor,
enhanced curcumin-induced autophagy and apoptosis by modifying
Bcl-2 expression and subsequent autophagosome formation in MCF-7
breast cancer cells. In vivo effect of curcumin and its
derivative (2E,6E)-2,6-bis(4-hydroxy-3-methoxybenzylidene)cyclohexanone
(BHMC) had been checked on 4T1 (triple-negative breast cancer
cell line) breast cancer cells challenged mice. A study showed
that curcumin and BHMC treated mice had low tumor burden,
mitotic cells, lung metastasis as well as regeneration capacity
compared to the untreated mice.
Effect of Curcumin on Prostate Cancer
A randomized, double-blind, controlled study evaluated the
effects of soy isoflavones and curcumin on serum PSA levels in
men. The authors of this study concluded that curcumin
presumably synergizes with isoflavones to suppress PSA
production. Curcumin against resistant prostate cancer, the induction of
apoptosis has been one of the modes of action of curcumin.
PI3/Akt pathway, which promotes cell growth, proliferation, and
survival, is inhibited by curcumin. Mechanistic studies, carried
out at subtoxic concentrations of curcumin in LNCap cells showed
that pretreatment with curcumin sensitized the cells towards
tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)
through inhibition of the NF-κB pathway of cell survival.
Castration-resistant prostate cancer cell (CRPC) line C4-2B,
showed a promising response to chemo-sensitization towards
remarkably low concentration dose of 10nM docetaxel on
pretreatment with a combination of 5 μM curcumin and 5 μM
nelfinavir, commendably without much adverse effect on primary
prostate epithelial cells. The molecular study revealed an
increase in pro-apoptotic markers caused by endoplasmic
reticulum (ER) stress and decrease in expressions associated
with PI3K/AKT survival pathway like phosphorylated-AKT. A summary of in vitro activities of curcumin against
various cancer cell lines has been compiled in Table 1. In a pilot phase II study, curcumin (6000 mg per day for 7
consecutive days) along with docetaxel and prednisone showed
therapeutic potential against castration-resistant prostate
cancer with good patient acceptability and tolerability
(Mahammedi et al. 2016). In vitro models revealed that,
curcumin treatment downregulated PGK1 via upregulation of
miR-143 alongside increased the expression of FOXD3, resulting
in inhibition of proliferation and migration of prostate cancer
cell (Cao et al. 2017). Curcumin treatment is known to induce
transferrin receptor protein 1 (TfR1) and iron regulatory
protein 1 (IRP1) expression which leads to induced autophagy and
apoptosis in castration-resistant prostate cancer cells (Yang et
al. 2017a). It has been reported that curcumin treatment
inhibited MT1-MMP and MMP-2 expressions in DU145 cells thus
reduced the metastasis and survival of prostate cancer cells
mediated by Notch-1 signaling cascade (Yang et al. 2017b).
Curcumin treatment induced the arrest of G0/G1 cell cycle phase
alongside inhibited the regulatory proteins cyclin D1 and CDK-2.
Besides, it upregulated the expression of p21, p27 and p53 while
downregulated Bcl-2 expression. Further, curcumin treatment is
known to activate caspase (3, 8 and 9) (Sha et al. 2016) while
decreased Akt, MMP (2 and 9), Bcl- 2, Bcl-XL and tumor volume in
prostate cancer (Jordan et al. 2016). Curcumin treatment is
reported to increase HDAC (1, 4 and 8), apoptosis, production of
ROS and Nrf- 2 expression, while decrease VEGF, HIF1-a, GSK-3b,
Akt, prostate-specific antigen (PSA) level, PSA mRNA expression,
HAT activity and cellular proliferation in LNCaP cell lines. The
available in vitro studies have shown that curcumin is able to
inhibit viability, proliferation, survival, migration/invasion,
and adhesion of various human prostate cancer cells. Curcumin
inhibited both androgen-sensitive and insensitive prostate
cancer cells by targeting a number of signaling cascades
responsible for regulating cellular function. The
antiproliferative, antisurvival, and antimigratory effects of
curcumin in prostate cancer cells may be due to the inhibition
of the Akt/mTOR, Ras/MAPK signaling pathways, decreased NF-κB
activation, enhanced proapoptoptic caspase and PARP cleavage,
and the inhibition of members of the antiapoptotic Bcl-2 family
of proteins. Curcumin was also able to induce cell-cycle arrest
and enhance autophagy in various prostate cancer cell lines. The
available in vivo studies have shown that curcumin
administration is able to inhibit the growth/volume, formation,
development, proliferation, and angiogenesis of prostate cancer
tumors while promoting apoptosis. These effects were observed in
mice xenografted with both androgen-sensitive and insensitive
prostate cancer cells. Curcumin’s inhibition of prostate tumor
growth and progression may be due to its inhibition of Akt
expression/activation, decreased NF-κB activation, inhibition of
the anti-apoptotic proteins Bcl-2 and Bcl-xL, increased
expression of the proapoptotic proteins Bax and Bak, and
enhanced PARP and caspase expression. These findings from in
vivo studies are in agreement with those from the in vitro
studies. The downregulation of cell proliferation, paired with
the enhanced activity of programmed cell death both in vitro and
in vivo, render curcumin an ideal candidate for the development
of novel anticancer pharmaceutical agents providing fewer
detrimental effects due to its low toxicity.
Androgendependent LNCaP
prostate cancer cells were injected subcutaneously into mice fed
with a 2% curcumin containing diet for up to 6 weeks. Curcumin
significantly increased the extent of apoptosis, as measured by
an in situ cell death assay, and caused a reduction in cell
proliferation, as measured by a BrdU incorporation assay.
Multiple studies have been done to evaluate the anticancer
effects of curcumin on androgen-sensitive as well as
androgen-resistant prostate cancer cell lines. T Dorai et al.,
2000, reported that curcumin can reduce the proliferation rate
to 20-30% compared to untreated LNCaP cells (androgen sensitive
prostate cancer cell-line). Asok Mukhopadhyay et al.
suggested that curcumin can cause tumor necrosis factor
(TNF)-induced apoptosis by suppressing NF-κB activation in the
prostate cancer cell. Similarly, curcumin also affects multiple
other proteins and pathways, such as c-Jun/activator protein 1
(AP-1), cyclin D1, CDK-4, phosphatidylinositol 3-kinase
(PI3K)/mechanistic target of rapamycin (mTOR)/E-twenty six
proto-oncogene 2 (ETS2) pathway to reduce proliferation, cell
growth in androgen-sensitive prostate cancer cell lines. Studies
have also shown the anticancer properties of curcumin on the
androgen-insensitive prostate cancer cell lines.
Curcumin-treated DU-145 prostate cancer cells showed reduced
expression of NF-κB in paired with less proliferation and
increased apoptosis. Curcumin additionally downregulated the
expression of nuclear transcription factor activator protein-1
(AP-1), composed of c-Fos and c-JUN. Many studies have analyzed
the effects of curcumin treatment in vivo on the mice
xenografted with various human prostate cancer cells. Thambi
Dorai et al., 2001, studied the effect of curcumin on athymic
nude mice implanted with LNCap cells. It showed a significant
increase in apoptosis and reduction in proliferation of LNCaP
cells demonstrated by the increased pycnotic brown staining
nuclei in situ.
Effect of Curcumin on Colorectal Cancer
Curcumin has demonstrated potential against colorectal cancer
in numerous clinical trials. Curcumin could be used to
avoid colorectal cancer (CRC) in diabetics with type 2 diabetes
by lowering leptin blood levels and increasing adiponectin
levels. In a dose-escalation study
that explored the pharmacology of curcumin in humans, 15
patients with advanced colorectal cancer refractory to
standard chemotherapies consumed capsules compatible with
curcumin doses of between 0.45 and 3.6 g/day for up to 4 months.
Levels of curcumin and its metabolites in plasma, urine, and
feces were analyzed. Curcumin and its glucuronide and sulfate
metabolites were detected in plasma in the 10 nmol/L range and
in urine. A daily dose of 3.6 g of curcumin caused 62% and 57%
decrease in inducible prostaglandin E2 production in blood
samples taken 1 h after the dose was administered on days 1 and
29, respectively. A daily oral dose of 3.6 g of curcumin was
recommended for the phase II evaluation in the prevention or
treatment of cancers outside the gastrointestinal tract. In
another study, patients were given curcumin capsules at three
different doses (3.6, 1.8, and 0.45 g/day) for 7 days . The
recoveries of curcumin in normal and malignant colorectal
tissues of patients receiving 3.6 g of curcumin were 12.7 ± 5.7
and 7.7 ± 1.8 nmol/g, respectively. In addition, two metabolites
of curcumin, curcumin sulfate and curcumin glucuronide, were
identified in the tissue samples. Trace levels of curcumin were
found in the peripheral circulation. The levels of M1G were also
decreased by curcumin treatment in malignant colorectal tissue.
The study concluded that a daily dose of 3.6 g of curcumin is
pharmacologically efficacious in colorectal cancer patients. Curcumin against resistant
colorectal cancer studies on human colorectal cancer cell line
HCT116 and its isogenic 5-fluorouracil (5-FU) resistant cell
line HCT116R in a 3D model showed that curcumin potentiated the
anti-proliferative activity of 5-FU against these cell lines
through apoptosis and inhibition of formation of colonies, with
suppression of NF-κB pathway. This synergistic combination
increased the percentage of apoptotic cells by 56% in HCT116R
cell lines. The molecular role of curcumin in apoptosis has
already been shown in another report where it intensified the
downregulation of anti-apoptotic BclxL and cell division
favoring cyclin D1 protein caused by 5-FU in HCT116 and
HCT116+ch3 (Complemented with chromosome 3) cell lines and
inhibiting activation of IkBα kinase and its phosphorylation.
Chemo-sensitization of drug-resistant cancer cell lines by
curcumin, towards a particular chemotherapeutic agent, has been
reported in one more investigation involving oxaliplatin
sensitive human colorectal adenocarcinoma HT29 Cells and its
oxaliplatin resistant derived sub-line HTOXAR3 cells, which
showed that combination of curcumin and oxaliplatin almost
reversed the oxaliplatin resistance. Clinically, curcumin
administration (3 g/day orally for one month) converted advanced
colon cancer derived regulatory T cells to T helper 1 cells via
increasing IFN-c production and repression of Foxp3 expression in
colon cancer patients (Xu, Yu, and Zhao 2017). In a
nonrandomized, open-label clinical trial, oral curcumin (2 g or
4 g per day for 30 days) administration reduced the number of
aberrant crypt foci and prevented the colorectal neoplasia
(Kunnumakkara et al. 2017; Carroll et al. 2011).
In vitro models
revealed that treatment with curcumin induced apoptosis,
arrested the cell cycle at the G1 phase, decreased the cell
population as well as inhibited the proliferation and mutation
of COLO 320DM cells (Dasiram et al. 2017). Additionally,
curcumin treatment stimulated 50AMP-activated protein kinase,
suppressed the phosphorylation of p65 NF-jB, downregulated
MMP-9 and urokinase-type plasminogen activator (uPA) expression
as well as reduced the binding ability of NF-jB DNA in LoVo and
SW480 cells leading to inhibition of colon cancer invasion (Tong
et al. 2016). Curcumin treatment downregulated chemokine
receptor 4 expression, upregulated naked cuticle homolog 2
expression and suppressed Wnt signaling. In addition, curcumin
treatment downregulated vimentin and upregulated E-cadherin
expression, which leads to inhibition of proliferation and
epithelial mesenchymal transition in SW620 human colon cancer
cells (Zhang et al. 2016d). Evidence suggested that curcumin
treatment downregulated the expression of p-glycoprotein
(Neerati, Sudhakar, and Kanwar 2013) and upregulated
PPAR-c protein (Liu et al. 2015), the potential mechanism by
which curcumin can be used for the treatment of colon cancer
(Neerati, Sudhakar, and Kanwar 2013). In a 30-day study in 44
men with lesions in the colon that sometimes turn cancerous, 4
grams of curcumin per day reduced the number of lesions by 40%.
In a nonrandomized, open-label clinical trial in smokers,
curcumin reduced the formation of aberrant crypt foci (ACF), the
precursor of colorectal polyps. Curcumin at 4 g/day
significantly reduced ACF formation. The reduction in ACF
formation by curcumin was associated with a significant fivefold
increase in post-treatment plasma curcumin/conjugate levels.
Curcumin was well-tolerated at both concentrations. These
findings demonstrated the effect of curcumin against ACF
formation in smokers. A study published in the International
Journal of Cancer found that curcumin compares favorably with
oxaliplatin as an antiproliferative agent in colorectal cell
lines. A study showed a profound reduction in the incidence of
colorectal carcinoma when curcumin is introduced. In another
recent study, curcumin was administered to patients with
colorectal cancer after diagnosis and before surgery. Curcumin
was given three times a day for 10–30
days. Curcumin administration decreased
serum TNF-α level, increased the number of apoptotic cells, and
enhanced the expression of p53 in tumor tissue. The authors of
this study concluded that curcumin treatment can improve the
general health of colorectal cancer patients via the mechanism
of increased p53 expression in tumor cells. In summary, the
studies discussed in this section suggest curcumin’s safety and
efficacy in patients with colorectal cancer.
Effect
of Curcumin on Pancreatic Cancer
| A single-blind, randomized,
placebo-controlled study from India was conducted to evaluate
the effects of oral curcumin with piperine on the pain and
markers associated with oxidative stress in patients with
tropical pancreatitis. Twenty patients with tropical
pancreatitis were randomly assigned to receive 500 mg of
curcumin with 5 mg of piperine or to receive placebo for 6
weeks, and the effects on the pattern of pain and on red blood
cell (RBC) levels of MDA and GSH were assessed. The results
indicated a significant reduction in the erythrocyte MDA levels
compared with placebo after curcumin therapy, with a significant
increase in GSH levels. The authors of this study concluded that
oral curcumin with piperine may reverse lipid peroxidation in
patients with tropical pancreatitis. Curcumin was found safe and
well-tolerated in a phase II clinical trial of patients with
advanced pancreatic cancer. Of the 25 patients enrolled in the
study, 21 were evaluable for response. Patients were given 8
grams of curcumin per day orally until disease progression, with
restaging every 2 months. No toxicities associated with curcumin
administration were noted in the patients. A downregulation in
the expression of NF–κB, COX-2, and pSTAT3 in peripheral blood
mononuclear cells of patients was observed after curcumin
intake. There was considerable interpatient variation in plasma
curcumin levels, and drug levels peaked at 22 to 41 ng/ml and
remained relatively constant over the first 4 weeks. The study
concluded that the oral curcumin is well-tolerated and, despite
limited absorption, has biological activity in some patients
with pancreatic cancer. An open-label phase II trial evaluated
the efficacy of curcumin in combination with gemcitabine against
advanced pancreatic cancer. Kanai et al. recently evaluated the
safety and feasibility of combinations of curcumin and
gemcitabine in 21 patients with gemcitabine-resistant pancreatic
cancer. Curcumin at 8 g/day in combination with gemcitabine was
safe and well-tolerated.
Effect of Curcumin on Bladder
Cancer
| In animal model, curcumin suppressed the invasion and growth
of bladder cancer via induction of apoptosis and arresting G1/S
phase transition in N-methyl-N-nitrosourea induced bladder tumor
in rats (Pan et al. 2017). Curcumin treatment suppressed the
N-methyl-N-nitrosourea-induced urothelial tumor in rats. In cell
lines studies, curcumin treatment is known to downregulate the
expression of insulin-like growth factor (IGF)-2 and reduces the
IGF1R and IRS-1 phosphorylation in T24 and UMUC2 bladder cancer
cells. In this regards curcumin functions through suppression of
IGF-2-mediated PI3K/AKT/mTOR signal transduction (Tian et al.
2017). Curcumin treatment reversed the transition of
epithelial-mesenchymal cells via reducing ERK5/AP-1 signaling
pathway in SV-40 human urothelial cells which might be the
potential drug candidate for prevention of bladder cancer (Liu
et al. 2017). In human bladder cancer cell lines, curcumin
treatment exert multiple effects like inhibition of MMP-2/9,
generation of ROS, upregulated the expression of HO-1, increased
the hypomethylation of the miR-203, upregulated the expression
of miR-203, inhibited Aurora A promoter activity, downregulated
histone H3 activation, induced G2/M phase cell cycle arrest,
decreased the expression of cyclin D1 and COX-2, decreased VEGF
level, decreased c-myc, decreased Bcl-2 expression,
downregulated Survivin protein, upregulated the expression of
p53 and Bax, induced fragmentation of DNA, downregulated cyclin
A expression and decreased NF-kB expression thereby inhibited
the cancer cell invasion, viability of cancerous cells and
growth (Imran et al. 2016; Saini et al. 2011).
Effect of Curcumin on
Blood Cancer (Multiple Myeloma and Leukemia), Lymphoma, and
other Hematological Malignancies
Curcumin against resistant leukemia showed inhibition in
growth and clonogenicity to curcumin treatment in dose and
time-dependent manner. Golombick et al. conducted a
single-blind, crossover pilot study to determine the effects of
curcumin on plasma cells and osteoclasts in patients with MGUS.
Curcumin decreased the paraprotein load in the ten patients with
paraprotein >20 g/L, and five of these ten had a 12% to 30%
reduction in paraprotein levels while receiving curcumin
therapy. In addition, 27% of patients receiving curcumin had a
>25% decrease in urinary N-telopeptide of type I collagen. The
study suggested the therapeutic potential of curcumin against
MGUS. Vadhan-Raj et al. evaluated the safety, tolerability, and
clinical efficacy of curcumin in 29 patients with asymptomatic,
relapsed, or plateau phase multiple myeloma. Curcumin was given
either alone (orally at 2, 4, 6, 8, or 12 g/day in two divided
doses) or in combination with piperine (10 mg in two divided
doses) for 12 weeks. Curcumin and piperine were well-tolerated,
with no significant adverse events. Furthermore, oral
administration of curcumin was associated with significant
downregulation in the constitutive activation of NF–κB and
STAT3, and it suppressed COX-2 expression in most of the
patients. These observations suggest the potential of curcumin
against multiple myeloma. In another study, curcumin showed an
IC50 value of 35.7 µM against KG1a 23.5 µM against Kasumi-1 on
96 hr exposure and completely stopped colony formation at 20 µM
concentration. The mechanistic investigations reflected the role
of curcumin in activation of Caspase-3, down-regulation of Bcl-2
mRNA expression and reduction in mitochondrial membrane
potential in addition to remarkable morphological changes like
cell shrinking and nuclear condensation, which are
characteristics of apoptosis. Another drug-resistant leukemia
cell line HL60 responded to curcumin with 50% growth inhibition
at 30 µM concentration. Cell cycle studies in this experiment
established apoptosis as the mechanism of action of curcumin and
arrest of the cell cycle in the S-phase was also reported in the
same study. Clinically, curcumin administration (5 g for 6
weeks) possessed potent chemosensitizing effect in chronic
myeloid leukemia patients, where the patients receiving both
curcumin and imatinib exhibited better prognosis with decreased
NO levels as compare to the patients receiving imatinib alone
(Ghalaut et al. 2012). In animal study, curcumin treatment
significantly decreased tumor growth in the chronic myeloid
leukemia xenograft mice via release of exosomes enriched miR-21
in plasma (Taverna et al. 2015). In cell line studies, curcumin
treatment upregulated apoptosis inducing factor, caspase-3,
cleaved PARP-1 while downregulated Bcl-2 resulting in induction
of apoptosis in lymphoblastic leukemia cells (Mishra, Singh, and
Narayan 2016). Curcumin incubation (10 lM, for 6 days) increased
the level of ROS, induced genomic instability, mediated reversal
of p15 promoter methylation and induced apoptosis in Raji cells
(Sharma et al. 2014). Curcumin treatment (40 mmol/L, for 48h)
downregulated the protein expression of nuclear NF- jB P65 as
well and its translocation alongside inhibited proliferation
of acute myeloid leukemia in KG1a and Kasumi-1 cells (Rao et al.
2015). Also, curcumin treatment (25 lM, for 24–48 h) arrested
cell cycle in the S-phase, increased the number of annexin
V-FITC(þ)/PI(-) cells and inhibited the proliferation of SHI-1
cells. In addition, curcumin upregulated FasL and downregulated
NF-jB, ERK, Bcl-2, MMP-2 and MMP-9 expressions. Further,
curcumin induced the activation of MAPK, p38, caspase-3 and JNK
resulted in inhibition of SHI-1 cell invasion (Zhu et al. 2016).
Curcumin treatment downregulated the expression of VEGF and
decreased the phosphorylation of AKT. Curcumin mediated
increased miR-196b levels caused downregulation of Bcr-Abl
expression in chronic myelogenous leukemia cells (Taverna et al.
2015). Curcumin incubation downregulated Mcl-1 expression and
associated with apoptosis in human myeloma cell lines
(Gomez-Bougie et al. 2015). Curcumin treatment simultaneously
inhibited RAF/MEK/ERK and AKT/mTOR pathway activation resulting
in induction of apoptosis and inhibition of proliferation in
human leukemia THP-1 cells (Guo et al. 2014). Curcumin
incubation increased the generation of intracellular ROS,
depletion of intracellular GSH, and activation of caspase
enzyme. Chu-Wen Yang et al. investigated the effect and mode of
action of curcumin on monocytic leukemia THP-1 cells, derived
from human acute monocytic leukemia. The authors demonstrated
that curcumin-induced THP-1 cell apoptosis through the
activation of c-Jun NH2-terminal kinase/extracellular
signal-regulated kinase/activator protein 1 (JNK/ERK/AP1)
pathways. Yi-Rong Chen et al. reported that curcumin affects the
mitogen-activated protein kinase kinase kinase 1/JNK
pathway by interfering with the signaling molecules like AP-1
and NF-κB as a possible mechanism of action. They speculated
that curcumin may affect the JNK pathway by interfering with the
signaling molecules at the same level or proximally upstream of
the MAPK kinase kinases (MAPKKKs) level. Yaowu Zhang et al.
showed curcumin can induce apoptosis in osteosarcoma MG63 cells
through the mitochondrial pathway. They reported that the
effects of curcumin-induced apoptosis in osteosarcoma cells were
associated with caspase-3 activation and reduced the levels of
Bcl-2 expression. Jia Rao et al. reported a similar function of
curcumin in AML cells. They showed that curcumin down-regulates
Bcl-2 and induces apoptosis in daunorubicin (DNR)-insensitive
CD34+ AML cell lines and primary CD34+ AML cells. Seong-Su Han
et al. reported that curcumin inhibited the proliferation of
BKS-2, an immature B cell lymphoma, more effectively than that
of normal B lymphocytes and caused the apoptosis of BKS-2 cells
in a dose- and time-dependent manner. The authors concluded that
curcumin downregulated the expression of survival genes early
growth response 1 (EGR-1), cellular myelocytomatosis (c-myc),
and Bcl-extra large (Bcl-XL) as well as the tumor suppressor
gene p53 in B cells as its possible mechanism of action. Shilpa
Kuttikrishnan et al. investigated the anticancer potential of
curcumin in acute lymphoblastic leukemia. The authors concluded
that curcumin suppresses B-pre-ALL cells' growth and
proliferation by inactivation of the PI3K/Akt signaling pathway.
Guo-Hua Zhu et al. reported that curcumin significantly induces
apoptosis but also partially suppresses invasion in SHI-1 cells
(acute monocytic leukemia cell line) in vitro. Their results
from polymerase chain reaction (PCR) and western blotting showed
that curcumin increased the FasL mRNA level; inhibited Bcl-2,
NF-κB, and ERK expression; and activated p38 MAPKs, JNKs, and
caspase-3. Zai-Xin Li et al. studied how curcumin affects the
proliferation of the Raji cells of Burkitt's lymphoma. Their
biochemical studies showed that cell apoptosis increases through
upregulation of Bid (BH3-interacting domain death agonist),
cytochrome C, and BAX, while oncogene c-Myc was downregulated
after curcumin treatment. Taken together, their results
suggested that mitochondrial damage induction was the main
mechanism of action of curcumin which led to apoptosis of the
Raji cells. In vivo effects of curcumin in the xenograft mouse
model showed its effective inhibition of tumor growth. All in
all, these results were suggestive of curcumin's growth
suppressing effect on Burkitt's lymphoma cells both in vivo and
in vitro system.
Effect of Curcumin on Cervical Cancer | Curcumin
administration (12,000 mg/day for 3 months) reduced the risk of
cervical cancer and is found to be safe and well tolerated
chemotherapeutic in phase I clinical trial (Cheng et al. 2001).
In animal model, curcumin suppressed nuclear b-catenin,
decreased oncogenic miRNA-21 and abrogated E6/E7 HPV expression
in orthotopic mouse model of cervical cancer (Zaman et al.
2016). Curcumin administration (1000 or 1500 mg/kg, for 30 days)
significantly downregulated the expression of VEGF, COX-2, EGF-R
and inhibited angiogenesis and tumor growth in cervical cancer
xenografts model of nude mice (Yoysungnoen-Chintana,
Bhattarakosol, and Patumraj 2014). In cell line studies,
curcumin treatment (13 mM) upregulated the expression of
early-onset breast cancer 1, O6- methylguanine-DNA
methyltransferase, mediator of DNA damage checkpoint 1,
p-H2A.XSer140 and p-p53 as well as induced translocation of
p-H2A.XSer140 and p-p53 from cytosol to nuclei, resulting in
chromatin condensation and induction of DNA damage in HeLa human
cervical cancer cells (Shang et al. 2016a). Curcumin activated
ATF6, PERK, IRE-1aand elevated the levels of ROS intracellularly
as well as induced apoptosis and inhibited the proliferation of
cervical cancer cells (ME180, C33A, HeLa and CaSki) (Kim et al.
2016a). Curcumin counteracts estradiol induced proliferation of
cervical cancer via induction of apoptosis in cervical cancer
cells (Singh and Singh 2011). Incubation with curcumin (20 mM,
for 72 h) reversed the hypermethylation and reactivation of the
RARb2 gene in cervical cancer cell lines (Jha et al. 2010).
Curcumin (50 or 100 mM, 24 h) dose dependently reduced the
phosphorylation of ERK, increased the activity of caspase 3 and
caspase 9, upregulated AIF, Bax, cytochrome while downregulated
Bcl-XL, Bcl-2 in cervical cancer cells. Curcumin treatment
downregulated the expression of cyclin D1, iNOS and COX-2 in
HeLa, SiHa and Ca Ski cells, and acts as an anti-proliferative
agent (Singh and Singh 2009).
Effect of Curcumin on Thyroid
Cancer
| In cell line studies, curcumin treatment upregulated
E-cadherin while downregulated vimentin and MMPs expressions
along with reduced metastasis, cell spreading and cell migration
in human papillary thyroid carcinoma cells. Curcumin suppressed
TGF-b1 mediated transcription, activation and secretion of
matrix metalloproteinases. It also inhibited TGF-b1 induced
Smad2 and Smad3 phosphorylation in human papillary thyroid
carcinoma BCPAP cells (Zhang et al. 2016a). Curcumin treatment
induced DNA damage in thyroid carcinoma BCPAP cells via
upregulation of H2A.X phosphorylation at Ser139 and ATM-mediated
activation of Chk2-Cdc25C-Cdc2 pathway. Moreover, curcumin
induced caspase mediated apoptosis in BCPAP cells (Zhang et al.
2016b). Curcumin downregulated the expression of HIF-1aand its
binding to hypoxia response element in K1 papillary thyroid
cancer cells. In addition, curcumin upregulated the expression
of E-cadherin, inhibited the activity of MMP-9 (Zhang et al.
2013a) and weakened K1 cells migration resulting in
anti-metastatic effect (Tan et al. 2015). Curcumin treatment
reduced the phosphorylation of PI3K and Akt pathway, and
downregulated the expression of MMP-1/7 and COX-2 leading to
inhibition of cell migration, growth and invasion of thyroid
cancer cells (FTC133) (Xu, Qin, and Liu 2014). Curcumin
instigate the production of ROS, reduce mitochondrial membrane
potential and altered intracellular calcium concentration
thereby mediate apoptotic induction in papillary thyroid cancer
cell line K1 (Song et al. 2012).
Effect of Curcumin
on Skin Cancer
| Curcumin
decreased the phosphorylation of IRS-1, ILGF-1 receptor, Akt,
4EBP1 and S6K in the mouse keratinocyte cells alongside exerted
significant anticancer activity against
7,12-dimethylbenz(a)anthracene (DMBA)-tetradecanoyl
phorbol-13-acetate induced skin cancer in mice (Kim et al.
2014). In in vitro studies, curcumin treatment is reported to
upregulate mmu-miR-205-5p expression, block proliferating cell
nuclear antigen, downregulate Bcl-2 expression and sup- press
JAK-2/STAT3 pathway which in turn induction of apoptosis and
inhibition of proliferation and invasion (Lelli, Pedone, and
Sahebkar 2017). Curcumin treatment arrested the G2/M phase of
cell cycle as well as induced autophagy in human melanoma cells
(A375 and C8161). In addition, curcumin reduced the activation
of P70S6K, and downregulated AKT and mTOR expressions which
might offer plausible target in the treatment of human melanoma
(Zhao et al. 2016a). In another study, curcumin decreased the
invasion of squamous cell carcinoma by suppressing STAT3
signaling pathway in A431 cells (Wu, Lu, and Cui 2015). Curcumin
induced the opening of mitochondrial permeability transition
pore and melanoma cell death in WM-115 melanoma cells (Qiu et
al. 2014). Curcumin inhibited NF-jB pro-survival pathway,
upregulated the p53 tumor suppressor protein and downregulated
Bcl-2 expression resulting in apoptosis and reversal of skin
cancer (Chinembiri et al. 2014).
Effect of Curcumin
on Brain Cancer
| Curcumin was shown to
have inhibitory effects on telomerase and induced telomere
shortening and apoptosis in brain tumor cells. Curcumin induced
growth inhibition and cell cycle arrest at G2/M in
medulloblastoma and glioblastoma cells. In various types of
cancers, curcumin was shown to selectively target cells that
express telomerase enzyme making these cells more vulnerable to
curcumin-induced cytotoxicity of cancer cells. Importantly, the
above-mentioned study revealed that the complex and diverse
action of curcumin, and its efficacy could depend on the cell
types used. The long-term studies on brain tumor cells
highlighted the use of curcumin as an adjuvant for cancer
therapy. Telomere shortening drives renal cell senescence and
leads to renal aging. Khaw and co-workers have demonstrated that
curcumin suppresses telomerase activity in brain tumor cells
which is associated with reduction in hTERT levels. Treatment
with curcumin induces a significant telomere shortening in brain
tumor cells suggesting its potential clinical application as
telomerase inhibitor and use of curcumin in adjuvant cancer
therapy. By contrast, in normal cells curcumin improves
viability by acting on telomerase when the cells have been
stimulated with toxic molecules. A study conducted on SK-N-SH
cells treated with curcumin improved cell viability. Normally,
hTERT was inhibited by Aβ1–42; shortened telomere could not
restore length, and then, there were plenty of apoptotic cells.
Treatment with curcumin could bind to Aβ1–42 and antagonize
neurotoxicity; thus, the expression of hTERT was upregulated,
shortened telomere restored length and the numbers of cells were
increased. Long-term studies on brain tumor cells underscore the
use of curcumin in adjuvant cancer therapy. Curcumin against brain cancer in vivo model of Human
glioma U-87 cells in athymic mice on intraperitoneal dose of
curcumin (120 mg/kg/day) showed less than 50% decrease in median
tumor volume in subcutaneous xenograft while in the orthotopic
model, the average life span of group receiving similar dose
increased by 12% as compared to the control group. In Female
SCID mice xenograft model, human primary medulloblastoma cells
(DAOY) were subcutaneously injected and after 30 days, the
animals were given oral gavage of curcumin (1 mg/kg) dissolved
in corn oil. The tumor growth inhibition in curcumin treated
group was significantly noticeable as compared to the control
group. The group of Smo/Smo transgenic medulloblastoma mice
receiving oral dose of curcumin was reported to have a median
survival time of 192 days as compared to the control group,
which had a median survival time of 144 days. This observation
is in agreement with earlier claims of ability of curcumin to
cross Blood Brain Barrier. Mechanistic insights in xenografted
human medulloblastom D425 cells in athymic mice showed
overexpression of p65 subunit of NF-κB and the curcumin treated
group showed tumor growth inhibition which can be partially
attributed to down regulation of p65 subunit. In another in vivo
investigation, human glioblastoma U87-MG cells-inoculated nude
mice were administered with 100 mg/kg per day of curcumin in
DMSO in Phosphate Buffer Saline through intra-tumoral
injections. After seven days, significant decrease in tumor size
was observed in curcumin treated group. Microscopic examination
post Acridine Orange staining showed increased acidic vesicular
organelles in curcumin treated cells with intact nuclei,
pointing towards curcumin-induced autophagy being responsible
for cell deaths.
Effect of Curcumin on Medulloblastoma and Neuroblastoma
Medulloblastoma is the common malignant brain tumor in
pediatrics. In animal model, curcumin inhibited tumor growth and
increased the survival rate in Smo/Smo transgenic
medulloblastoma mice (Lee et al. 2011). In cell line studies,
curcumin treatment arrested G2/M phase of cell-cycle, activated
GSK-3band suppressed Wnt/b-catenin path- way resulting in
inhibition of proliferation in DAOY medul- loblastoma cell line
(He et al. 2014). Curcumin treatment upregulated the PTEN gene
expression and downregulated the expression of E2F1, CDK2 and
cyclin E1 gene resulting in growth arrest at G2/M phase in
medulloblastoma cells. In addition, curcumin treatment increased
caspase-3/7 activity, overexpressed Bax while downregulated
Bcl-2, Bcl-Xl and surviving expression, which leads induced
apoptosis of human medulloblastoma cells (Bangaru et al. 2010).
Curcumin treatment inhibits telomerase activity and gene
expression of hTERT resulting in telomere shortening in
medulloblastoma cell lines (A172, KNS60, U251MG and ONS76) (Khaw
et al. 2013). Curcumin phosphorylates Cdc27, a component of the
anaphase promoting complex/ cyclosome, which is known to
ubiquitinate securing and cyclin B, resulting in proteolysis and
apoptosis of DAOY medulloblastoma cell (Lee and Langhans 2012).
Further, it was reported that, curcumin treatment induced
apoptosis and cell cycle arrest possibly through downregulation
his- tone deacetylase 4 and enhanced tubulin acetylation.
Curcumin treatment inhibited the sonic hedgehog-glioma
associated oncogene-1 pathway via downregulating the protein
expression of sonic hedgehog ligand, and its most important
downstream targets glioma associated oncogene-1 and patched-1
receptor. Furthermore, curcumin reduced the levels of b-catenin,
N-myc, C-myc, cyclin D1 and induced apoptosis in DAOY
medulloblastoma cells (Elamin et al. 2010).
Effect of
Curcumin on Ovarian
Cancer | Curcumin exhibits the important anti-cancer
activity in ovarian cancer via the pro-apoptotic function.
Curcumin inhibited ovarian cancer cell proliferation and
promoted apoptosis, and first confirmed it was associated with
the regulatory network of circ-PLEKHM3/miR-320a/SMG1. Liu et al.
reported that curcumin could constrain ovarian cancer cell
proliferation and facilitate apoptosis by inhibiting autophagy
and AKT/mTOR/p70S6 pathway. Yen et al. suggested that curcumin
could suppress ovarian cancer cell colony formation via blocking
the Wnt/β-catenin pathway. These reports indicated the
anti-cancer property of curcumin in ovarian cancer treatment. In
the past research, curcumin had been found to improve the
radiosensitization of nasopharyngeal carcinoma through
regulating the circRNA network. Xu et al. suggested that
curcumin could suppress non-small cell lung cancer progression
by regulating circ-PRKCA. Circ-PLEKHM3 was downregulated in
ovarian cancer, and its expression could be promoted by
curcumin. Function analysis showed that circ-PLEKHM3
overexpression could aggravate curcumin function by suppressing
cell proliferation, triggering apoptosis and reducing
tumorigenesis in ovarian cancer. These data revealed that
curcumin might regulate ovarian cancer progression by promoting
circ-PLEKHM3. In addition, the anti-cancer role of circ-PLEKHM3
was confirmed in study, which was consistent with the previous
study. A previous report displayed the circ-PLEKHM3 acted as
miR-9 sponge to regulate ovarian cancer progression. MiR-320a
mimic reversed the regulation of circ-PLEKHM3 on
curcumin-mediated ovarian cancer cell proliferation and
apoptosis, further confirming that circ-PLEKHM3 sponged miR-320a
to participate in ovarian cancer progression. The study also
validated the carcinogenic role of miR-320a in ovarian cancer,
which was consistent with previous reports. These data indicated
the importance of circ-PLEKHM3/miR-320a axis for curcumin in
ovarian cancer development. Curcumin could up-regulate SMG1
expression via modulating circ-PLEKHM3/miR-320a axis. Curcumin
could suppress proliferation and promote apoptosis in ovarian
cancer, possibly via regulating circ-PLEKHM3/miR-320a/SMG1 axis.
This research might propose a novel mechanism for understanding
the function of curcumin in ovarian cancer.
Effect of
Curcumin on Liver Cancer | Yu and his colleagues
evaluated the role of curcumin in inhibiting the human hepatoma
SMMC-7721 cells significantly by promoting apoptosis via
modulation of Bax/bcl-2 (Yu et al., 2011). Apoptosis was
associated with increases in p53 levels as well as its
DNA-binding ability, along with protein expression of Bax.
Phosphorylation of CDC27 (cell division cycle 27) is the main
mechanism of anticancer efficacy of curcumin by obstructing cell
growth and proliferation in an apoptotic pathway, leading to the
death of the cells (Lee and Langhans, 2012). According to Li and
his colleagues, in human hepatoma cell lines such as HepG2 and
HCCLM3, suppression of miR-21 improved anticancer action of
curcumin like cell growth suppression, apoptosis via upregulated
target gene, and TIMP3 expression, and the mechanism may refer
to TGF-β1/smad3 signaling pathway inhibition (Li J. et al.,
2020). Curcumin inhibits cancer through modulating several
signaling pathways and molecular targets, including
TGF-β1/smad3, IGF, PI3K/Akt, Wnt/β-catenin, and vascular
endothelial growth fact (VEGF) (Figure 7) (Mohebbati et al.,
2017).
Effect of
Curcumin on Other
Cancer | Curcumin treatment significantly inhibited gastric
carcinoma. Curcumin therapy of Burkitt’s lymphoma cell lines in
combination with ionizing radiation shows that it boosts
lymphoma cells’ susceptibility to ionizing radiation-induced
apoptosis and improves cell cycle arrest at the G2/M phase.
Curcumin and L-ASP show synergism in patients with blood and
bone marrow malignancy (Jiang et al., 2015). Curcumin also
hinders the cellular growth of uterine leiomyosarcoma and
reduces the spread of castrate-resistant disease and human
leiomyosarcoma cells via modulating the AKT-mammalian target of
rapamycin pathway for inhibition (Wong et al., 2011). Curcumin
extract’s potential in decreasing tumors induced chemically was
investigated. It was documented that curcumin is useful in
reducing papilloma development throughout carcinogenesis and
progression. Dietary curcumin can reduce the number of papilloma
that promoted skin tumor, which was explored by Limtrakul et al.
(2001) as ras-p21 and fos-p62 oncogene expression was decreased
dose-dependently by curcumin. In addition, curcumin prevents the proliferation of
uterine leiomyosarcoma via induction of apoptosis, autophagy,
ERK 1/2 activity and fragmentation of DNA in gastric carcinoma
cells (Imran et al. 2016). Curcumin treatment suppressed
JAK-STAT signaling thus reducing tumor cell growth in ovarian
(OVCA 420 and OVCA 429) and endometrial (RL95-2 and Ishikawa)
cancer cell lines (Saydmohammed, Joseph, and Syed 2010).
Curcumin down-regulated the expression of IL-6, IL-11 and NF-kB
which leads to induce apoptosis of fibrosarcoma cells resulting
in anticancer activity against bone cancer (Kondo et al. 2001;
Kwak et al. 2006). Curcumin induced cell cycle arrest in G2/ M
phase, apoptosis and cytotoxicity in squamous carcinoma cells as
well as reduced tumor volume in head and neck cancer (Borges et
al. 2017). Curcumin treatment reversed the migration and
proliferation of hepatic carcinoma by downregulating the
expression of HIF-1a. In addition, curcumin reduced the level of
MMP-2 and MMP-9 as well as decreased the phosphorylation of p38,
which is associated with suppression of cancer invasion and
migration in hepatic carcinoma. Additionally, curcumin
treatment exhibited anti-proliferative effect in MHCC97H liver
cancer cells through generation of ROS, apoptosis and activating
toll like receptor -4/MyD-88 pathway (Imran et al. 2016; Liang
et al. 2014). Curcumin treatment significantly upregulated the
expression of p21/CIP1 and p27/KIP1 CDK, and downregulated the
expression of cyclin D1 resulting in decreased proliferation of
pancreatic cancer cells. Apart from this, curcumin induced
apoptosis via downregulating the ratio of Bcl-2/Bax and
increasing the activation of caspase-9/3 in pancreatic cancer
cells. Curcumin treatment inhibited PI3K/ Akt pathway and
induced forkhead box O1 in Panc-1 pancreatic cancer cells
leading to apoptosis (Zhao et al. 2015). Curcumin suppressed the
oral tumor volume, numbers of dysplasic lesions, papillomas and
squamous cell carcinoma (Imran et al. 2016). Interestingly,
curcumin treatment has potential for many cancer types like
esophagus cancer, testicular cancer, sarcoma and lymphoma
(Kunnumakkara et al. 2017). Curcumin has been reported to have
pharmacological efficiency towards multiple other cancer types
like gastric, colorectal, liver, and osteosarcoma. Xiang Zhou et
al. reported that curcumin, in combination with oxaliplatin and
5-fluorouracil (5-FU), exhibited synergistic inhibitory effect
in xenograft gastric tumor (BGC-823 cancer cells) via
downregulation of Bcl-2 and cleavage of caspase-3 and PARP
through upregulation of BAX [81]. Gizem Calibasi-Kocal et al.
reported the dose-dependent chemopreventive role of curcumin in
HCT-116 and LoVo cells (human colon cancer cell lines) possibly
through inhibition of NF-κB and/or activation of caspase-3 and
caspase-9. Biqiong Ren et al. demonstrated the antiproliferative
role of curcumin on liver cancer and reported its mechanism of
action through inhibition of the heat shock protein 70-toll like
receptor 4 (HSP70-TLR4) signaling pathway. Duk Su Lee DS et al.
demonstrated curcumin-induced p53 upregulation, cell cycle
arrest at gap-1/synthesis (G1/S) and G2/S phase, and caspase-3
activation in human osteosarcoma cells. Curcumin has been
reported to possess antiproliferative activity towards
fibrosarcoma, a rare malignant tumor of the fibrous connective
tissue around the bones. MR Guimarães et al. reported that
curcumin was able to inhibit cytokine gene expression in
diseased periodontal tissue. They discovered curcumin-induced
inactivation of IL-6, and IL-11 in a dose-dependent manner. |
|
| How may
Curcumin work against Rheumatoid arthritis and Osteoarthritis? |
|
|
The
potential of curcumin against arthritis was first reported in
1980 in a short-term, double-blind, crossover study involving 18
young patients with rheumatoid arthritis. In this study,
curcumin’s efficacy was compared with that of the prescription
drug phenylbutazone. Patients were randomly assigned to receive
either curcumin (1.2 g/day) or phenylbutazone (0.3 g/day) for 2
weeks. Curcumin was well-tolerated, had no adverse effects, and
exerted an anti-rheumatic activity identical to that of
phenylbutazone as shown by improvement in joint swelling,
morning stiffness, and walking time. Curcumin can reduce joint
inflammation and alleviate pain symptoms, mainly due to its
anti-inflammatory and cartilaginous protective effects. In
primary cultured chondrocytes, curcumin inhibited the mRNA
expression of pro-inflammatory mediators IL-1β and TNF-α, MMPs
1.3 and 13, and ADAMTS5, and upregulated the chondroprotective
transcriptional regulator Cbp/p300 interacting transactivator
with ED-rich tail 2. Curcumin reduces the synthesis of
inflammatory mediators, such as TNF-α, IL-17, IL-1β,
transforming growth factor-β (TGF-β), and cyclooxygenase-2 and
reduces the cartilage and synovial inflammation of rat models of
arthritis induced by lipopolysaccharide, Collagen II and
Monoiodoacetic acid. Curcumin exerts an anti-inflammatory effect
by inhibiting TLR4 pathway and its downstream NF-κB signaling
pathway. Activation of NF-κB pathway not only down-regulates
pro-inflammatory factors, but also inhibits the expression of
matrix degrading enzymes. Curcumin inhibited IL-1β-induced MMP-1
and MMP-3 production by inhibiting AP-1 and NF-κB signaling
Pathway activation. Arthritis is an inflammatory autoimmune
disease characterized by chronic inflammation of the synovial
joint that can lead to severe joint injury. IL-10 plays an
important role in the development of rheumatoid arthritis.
Curcumin has anti-inflammatory effect and can regulate TLR-4
receptor and its downstream pathway.65 Curcumin can
down-regulate the levels of TNF-α, IL-1β, IL-6, IL-12, IL-15,
and IL-8 in macrophages, and up-regulate the level of IL-10.
Curcumin effectively alleviates MSU-induced inflammatory
response by inhibiting TLR4/NF-κB signaling pathway and NLRP3
inflammasome activity. Curcumin is a natural anti-inflammatory
drug. Numerous preclinical studies have demonstrated its
beneficial effect on arthritis. Clinical trials focused on the
treatment of knee osteoarthritis. In a clinical trial of
turmeric extract in the treatment of knee osteoarthritis,
turmeric extract inhibited inflammation and improved clinical
symptoms, as well as reduced IL-1β and oxidative stress.
Turmeric extract was more effective than placebo for knee pain.
Motahar Heidari-Beni et al produced an herbal formulation
consisting of turmeric extract, black pepper and ginger. In
patients with knee osteoarthritis, this compound raises
prostaglandin E2 (PGE2) levels similar to naproxen. In a
randomized, pilot study, 45 patients diagnosed with arthritis
were randomized into three groups with patients receiving
curcumin (500 mg) and diclofenac sodium (50 mg) alone or their
combination. Results show that curcumin administration showed
the significantly improvement in overall Disease Activity Score
and American College of Rheumatology compare with diclofenac
sodium. Clinical trials of curcumin in the treatment of
arthritis have produced promising results. Currently
curcumin-containing dietary supplements are widely used for
joint health.
In another recent study,
curcumin alone (0.5 g) and in combination with diclofenac sodium
(0.05 g) was found to be safe and effective in 45 patients with
rheumatoid arthritis. Furthermore, the level of CRP was
suppressed in these patients after curcumin administration. Several studies have shown the anti-arthritic effects of
curcumin in humans with osteoarthritis and rheumatoid arthritis
(RA). In a randomized double-blind placebo-controlled trial, 40
subjects with mild-to-moderate degree knee osteoarthritis were
randomly assigned to receive either curcuminoid (500 mg/day in
three divided doses; n = 19) with 5 mg piperine added to each
500-mg dose or a matched placebo (n = 21) for six weeks. There
were significantly greater reductions in the visual analog scale
(VAS) (p < 0.001), Western Ontario and McMaster Universities
Osteoarthritis Index (WOMAC) scores (p = 0.001), and Lequesne’s
pain functional index (LPFI) (p = 0.013) scores in the treatment
group compared with the placebo group. This suggests that
curcumin may offer an alternative to NSAIDS for patients with
osteoarthritis seeking treatment but experiencing negative side
effects. This was supported by results from a pilot study
showing that a dose of 2 grams of curcumin had an analgesic
effect in subjects with acute pain but without a diagnosis of
osteoarthritis . At this dose, the activity was higher than that
associated with 500 mg of acetaminophen, while a lower dose (1.5
g, 300 mg of curcumin) gave only transient and often inadequate
relief of pain, indicative of suboptimal therapeutic plasma
concentrations. This supports the use of 2 g (higher than
needed for inflammation) curcumin for relief of pain as a
potential alternative to NSAIDS. Regardless of the mechanism by
which curcumin elicits its effects, it does appear to be
beneficial to several aspects of osteoarthritis , as suggested
by a recent systematic review and meta-analysis that concluded:
“This systematic review and meta-analysis provided scientific
evidence that 8–12 weeks of standardized turmeric extracts
(typically 1000 mg/day of curcumin) treatment can reduce
arthritis symptoms (mainly pain and inflammation-related
symptoms) and result in similar improvements in the symptoms as
ibuprofen and diclofenac sodium. Therefore, turmeric extracts
and curcumin can be recommended for alleviating the symptoms of
arthritis, especially osteoarthritis”. A recent study out of Japan evaluated its relationship with the
inflammatory cytokine known to be involved in in the
rheumatoid arthritis
process. Scientists discovered that curcumin “significantly reduced” these
inflammatory markers. In fact its anti-inflammatory qualities are so strong
a 2007 study compared
curcumin and cortisone and found they were equal in potency. A few
studies have found that curcumin can reduce pain from rheumatoid arthritis
or osteoarthritis, sometimes as much as anti-inflammatory drugs. An
Iranian clinical trial in
Phytotherapy Research in 2014 found
that curcumin taken for six weeks, improved symptoms of knee osteoarthritis,
compared to a placebo. Most
pharmaceutical anti-inflammatories are contraindicated to use over the
long-term, but turmeric is not only safe but beneficial for your overall
wellbeing. Curcumin's anti-inflammatory properties also make it a strong candidate
for treating inflammatory diseases such as osteoarthritis.
A 2014 study in the
Clinical Interventions in Aging found that curcumin extracts "were as
effective as ibuprofen for the treatment of knee osteoarthritis." All current drugs approved for arthritis have anti-inflammatory
activity. Anti-TNF (tumor necrosis factor) therapy has been approved for this
disease. Curcumin has been shown to both suppress the TNF production, block
the action of TNF, and have
activity against arthritis.
When inflammation is reduced, the added benefit is
pain relief. A double-blind, crossover study showed that Curcumin may be effective
in relieving pain and improvements in morning stiffness, walking time, and joint
swelling. A preliminary intervention trial that compared curcumin with a
nonsteroidal anti-inflammatory drug (NSAID) in 18 patients with rheumatoid
arthritis (RA) found that improvements in morning stiffness, walking time,
and joint swelling after two weeks of curcumin supplementation (1.2 g/day)
were comparable to those experienced after two weeks of phenylbutazone
(NSAID) therapy (300 mg/day). In a more recent randomized, open-label study
in 45 RA patients, supplementation with a mixture of all three major
curcuminoids (0.5 g/day for eight weeks) was found to be as effective as
diclofenac (NSAID; 50 mg/day) in reducing measures of disease activity,
tenderness, and swelling joints.
Several
studies
have shown
curcumin’s ability to reduce pain, stiffness, and swelling
in joints afflicted by arthritis. The
Arthritis Foundation suggests that those with arthritis who
wish to seek relief take capsules of curcumin powder, between
400 mg to 600 mg, three times a day. Given that curcumin is a
potent anti-inflammatory compound, it makes sense that it may
help with arthritis. Several studies show this to be true. In a
study in people with rheumatoid arthritis, curcumin was even
more effective than an anti-inflammatory drug (42). Many other
studies have looked at the effects of curcumin on arthritis and
noted improvements in various symptoms. An
in vitro
and ex vivo study
found that curcumin has anti-platelet and prostacyclin
modulating effects compared to aspirin, indicating it may have
value in patients prone to vascular thrombosis and requiring
anti-arthritis therapy. In a randomized, pilot study, curcumin
administration (500 mg, b.i.d., p.o., for 8 weeks) reduced
Disease Activity Score in rheumatoid arthritis without any
adverse events. In addition, the effect of curcumin was better
than the patients receiving diclofenac sodium (Chandran and Goel
2012). In animal model, curcumin administration (100 mg/kg
orally for two weeks) showed anti-arthritic activity by
augmenting the generation of somatostatin in the small intestine
of Freund’s complete adjuvant induced arthritic rats (Yang et
al. 2015). Curcumin (50 mg/kg, i.p.) attenuated the severity and
progression of collagen induced arthritis in DBA/1 J mice by
decreasing the production of B cell-activating factor belonging
to the TNF family in spleen cells and serum as well as reduction
of serum IL-6 and IFNc(Huang et al. 2013). It reduced the pannus
formation process that produced through articular cartilage of
collagen induced arthritic rats (Kamarudin et al. 2012). In in
vitro studies, curcumin treatment (2.5–10 mmol for 14 days)
inhibited the osteoclastogenic potential of peripheral blood
mononuclear cells obtained from patients with rheumatoid
arthritis by decreasing stimulation of ERK 1/2, c-Jun N-terminal
kinase, p38 and downregulating nuclear factor of activated T
cells (NFATc1), receptor activator of NF-jB and c-Fos
expression, and reduce bone deterioration during rheumatoid
arthritis (Shang et al. 2016b). Curcumin treatment efficiently
blocked phorbol 12-myristate 13 acetate and IL-1b-induced
upregulation of IL-6 expression in MH7A cells and
Fibroblast-like synoviocytes. In addition, it inhibited NF-jB
activation, induced ERK1/2 dephosphorylation, exerted strong
anti-inflammatory activity and induced apoptosis in
fibroblast-like synoviocytes, which might use as a natural
remedy for the management of rheumatoid arthritis (Kloesch et
al. 2013). Mechanistically, curcumin blocks certain cytokines
and enzymes that lead to inflammation, and this sheds light on
the possibility of curcumin for the treatment of rheumatoid
arthritis. Osteoarthritis is the most common type of arthritis,
which is characterized by pain, tenderness, bone spurs,
stiffness, and loss of function in the joints (Farzaei et al.
2015). In a randomized, double-blind, placebo-controlled
prospective study, chronic administration of curcumin (180
mg/day, p.o., for 8 weeks) significantly reduced knee pain in
osteoarthritic patients as compared to the placebo group
(Nakagawa et al. 2014). Curcumin treatment showed protection
against osteoarthritis by inhibiting the release of inflammasome
NLRP3, followed by downregulation of IL-1b, TNF-a and cleaved
caspase-1 in surgical mouse osteoarthritis model (Sun et al.
2017). Mechanistically, curcumin reduced MMP-2, MCP-1,
L-selectin, advanced oxidation protein product levels,
suppressed the release of proteoglycans, expression of
cyclooxygenase, prostaglandin E2 and inflammatory cytokines
while increased CD47 levels in chondrocytes (Liu et al. 2016;
Chin 2016). |
|
How may Curcumin work against
Gastrointestinal and Inflammatory bowel disease such as Crohn's disease, Ulcerative Colitis, Irritable bowel syndrome,
gastritis, dyspepsia, gastric and peptic ulcers? |
|
Six hundred milligrams of curcumin
five times a day for 12 weeks to individuals with peptic ulcers
could prevent ulcer development. Abdominal pain along with other
symptoms has greatly decreased with curcumin within 1–2 weeks.
Kim et al. (2005) found that orally administered ethanolic
C. longa extract decreased stomach acid, gastric juice
secretion, and ulcer initiation in male rats by inhibiting H2
histamine receptors, which is similar to the effects of
ranitidine. Similarly, the antiulcer action of
C. longa
ethanolic extract was seen as it lowers ulcer index in addition
to stomach acidity significantly. C. longa extract also
suppressed hypothermic-restraint stress depletion of stomach
wall mucus and diminished the severity of necrotizing
agent-induced lesions. Curcumin significantly protects against severe colitis by inhibiting activation of NLRP3
inflammasomes and production of IL-1β, resulting in improved
weight loss, reduced disease activity index and increased colon
length. Curcumin can inhibit the production of
pro-inflammatory factors which is beneficial to improve
intestinal inflammation in patients with IBD. Curcumin can
effectively induce and maintain symptom relief in patients with
ulcerative colitis, reduce inflammatory markers and improve the
quality of life of patients. Curcumin is derived from natural
products, with high safety, has the capacity for
anti-inflammatory, antioxidant, and regulating autophagy and gut
microbiota. Curcumin is a safe and effective adjuvant agent in
the treatment of IBD. In patients with IBD, curcumin has a
beneficial effect on clinical symptoms, endoscopic relief,
reduction of oxidative stress or inflammatory markers.
Alternatively, curcumin can also play a beneficial role in a
more common intestinal disease. Irritable bowel syndrome is a
functional bowel disorder that classically presents with
symptoms of abdominal pain, bloating, and altered bowel habits
of diarrhea or constipation. The Irritable Bowel Syndrome-
symptom severity score (IBS-SSS) was used to evaluate the effect
of curcumin on patients with IBS. Curcumin can effectively
improve IBS-SSS, abdominal pain and other symptoms, and improve
the quality of life of patients. Research suggest that the
beneficial effects of curcumin on IBS may be due to its
anti-inflammatory effect. Because of its scientifically
evidenced characteristics to interfere with a variety of signal
transduction pathways, transcription factors, and cellular
processes, curcumin can potentially be applied in the treatment
of many diseases (inflammatory disorders in particular). In this
context, curcumin has been used to treat gastrointestinal
diseases such as indigestion, flatulence diarrhea, and even
gastric and duodenal ulcers. Kwiecien and colleagues summarize
in their review curcumin’s protective effects against esophageal
and gastric disorders. In addition, curcumin is potentially
efficacious against intestinal inflammatory diseases. Burge and
colleagues discuss the beneficial effects of curcumin on the
microbiome, its antimicrobial properties, changes in cytokine
profiles, and alterations to immune cell maturation and
differentiation. The combination of all these molecular actions
makes curcumin a promising candidate to treat intestinal
inflammatory diseases like necrotizing enterocolitis, Crohn’s
disease, and ulcerative colitis. Crohn’s disease is a pro-inflammatory disease. All current drugs approved
for this disease have anti-inflammatory activity. Anti-TNF therapy has been
approved for this disease. Curcumin has been shown to both suppress the TNF
production and the TNF action. Several clinical trials suggest that curcumin can
help people with this inflammatory bowel disease.
Clinical Gastroenterology and Hepatology featured a study in 2015
which found that in people with mild to moderate ulcerative colitis who took
standard medication (mesalamine), the addition of a high-dose curcumin
supplement helped half of them achieve remission after four weeks; none of
those given a placebo benefited. Curcumin taken orally has been shown to have
activity against inflammatory bowel disease. Study results
suggest that Curcumin could have a protective role in ulcerative colitis via
regulation of oxidant/anti-oxidant balance and modulation of the release of some
inflammatory endocoids, namely TNF-alpha and NO.
Curcumin maintenance
therapy for ulcerative colitis: randomized, multicenter, double-blind,
placebo-controlled trial. The development of DSS-induced
colitis was significantly attenuated by curcumin. Inhibition of p38 MAPK
signaling by curcumin could explain the reduced COX-2 and iNOS immunosignals and
the nitrite production in colonic mucosa, reducing the development of chronic
experimental colitis. In addition, Curcumin seems promising with regards to remission in patients with quiescent Ulcerative
Colitis. Preliminary evidence suggests that curcumin might be useful as an
add-on therapy to control disease activity. One multicenter, randomized,
double-blind, placebo-controlled study has examined the efficacy of curcumin
enema (2 g/day) in the prevention of relapse in 82 patients with quiescent
UC . Six-month treatment with curcumin significantly reduced measures of
disease activity and severity and resulted in a lower relapse rate than with
placebo in subjects on standard-of-care medication (sulfasalazine or
mesalamine). In another randomized controlled trial in active UC patients
treated with mesalamine, the percentage of patients in clinical remission
was significantly higher after a one-month treatment with oral curcumin (3
g/day) than with placebo.
Curcumin in
Combination With Mesalamine Induces Remission in Patients With
Mild-to-Moderate Ulcerative Colitis in a Randomized Controlled Trial.
Another
study conducted in the UK revealed that those with IBS who
took two capsules of turmeric every day over the course of eight
weeks experienced less abdominal pain and had more consistent
bowel movements. A recent study from the
American Gastroenterological Association suggests that
curcumin may help ease ulcerative colitis, a form of
inflammatory bowel disease that
causes ulcers in the digestive tract. Turmeric has been known
for a long time to help with digestive problems. For example, it
helps very well with bad digestion of fats. But even if you
suffer from irritable bowel syndrome or Crohn’s disease,
turmeric can mean a great deal to you. This is partly because it
prevents inflammation in the intestinal wall. Curcumin can also
be a solution for people with a stomach ulcer. Curcumin has a
significant role in cases of gastric ulcers. An open, phase II
trial was performed on 25 patients with endoscopically diagnosed
gastric ulcer. Participants were provided 600 mg powdered
turmeric, five times daily. After 4 weeks, ulcers had completely
healed in 48% patients. The success rate increased over time,
with 76% being ulcer free after 12 weeks of treatment. No
significant adverse reactions or blood abnormalities were noted.
In a clinical study, five patients with a stomach ulcer were
given five times 600 mg of curcumin every day for 12 weeks.
Almost half of them had no stomach ulcers after four weeks, and
by the end of the study, the gastric ulcer had disappeared by
76%. In a multi-centered, double-blind, placebo-controlled
trial, curcumin treatment (1 g after breakfast and 1 g after the
evening meal with mesalamine or sulfasalazine for 6 months)
appeared to be a safe and promising drug candidate for
maintaining remission in ulcerative colitis patients (Hanai et
al. 2006). Holt et al. (2005) carried out a pilot study to see
how curcumin therapy affected IBD patients who had earlier
received standard UC or CD therapy. Curcumin with standard
treatment exerts more beneficial effects than placebo plus
conventional UC treatment in maintaining recovery, according to
Hanai et al. (2006). Bundy et al. (2004) examined that abdominal
pain or discomfort score was lowered significantly by 22% and
25% in the one- and two-tablet group volunteers, respectively,
and revealed the role of C. longa on IBS pathology. In animal study, curcumin administration reversed
inflammation of the colonic mucosa, restored colonic length, and
reduced colonic weight and colonic damage. In addition, curcumin
increased the number of T regulator (Treg) cells while
suppressed the secretion of IL (2, 6, 12 and 17) and TNF-a.
Curcumin is known to downregulate the expression of
co-stimulatory molecules CD254 [RANKL], CD54 [ICAM-1], CD205,
CD256 [RANK], TLR4 and CD252[OX40 L] against 2, 4,
6-trinitrobenzene sulfonic acid induced colitis in mice (Zhao et
al. 2016b). In a recent experimental study, curcumin
administration demonstrated therapeutic potential through
downregulation of colonic TNF-a, myeloperoxidase (MPO), p-38MAPK
and p-p38MAPK expressions in mouse murine ulcerative colitis
model (Khoury et al. 2015). Curcumin treatment is known to
reduce interferon (IFN)-c, COX-1, COX-2, TNF-a, NF-jB and iNOS
expression. Further, it was reported that curcumin treatment
reduces inflammation of colon due to inhibition of chemokinesis
and neutrophil chemotaxis (Wan et al. 2014). Moreover, curcumin
mitigated inflammatory bowel disease via influencing MAPK, ERK
pathways, increasing antioxidants, inducing free radical
scavenging and MPO inhibition (Baliga et al. 2012).
Mechanistically, curcumin treatment reduced ulcerative colitis
by inhibiting neutrophil chemotaxis, suppressing the secretion
of inflammatory cytokines and inducing antioxidant effects. In a
pilot study, administration of curcumin (350 mg, t.i.d. for 1
month followed by 350 mg q.i.d. for another 2 month) reduced the
inflammatory response in Crohn’s disease condition. In addition,
it reduced the erythrocyte sedimentation rates and Crohn’s
Disease Activity Index in patients (Holt, Katz, and Kirshoff
2005). Oral administration of curcumin (40 mg/kg, for 21 days)
reversed the visceral nociceptive response to graded intensity
of colorectal distension and pellet output associated with
chronic acute combined stress mediated depressive and anxiety
like behaviors in rats. Mechanistically, curcumin treatment
increased the levels of serotonin, BDNF and pCREB in the
hippocampus, while these levels were reduced in the colonic of
chronic acute combined stressed rats (Yu et al. 2015). The
5-HT1A receptor is known to be involved in the mode of action of
curcumin for the management of visceral hypersensitivity in rats
with irritable bowel syndrome. In addition, curcumin
administration causes remarkable decrease in visceromotor
response to colorectal distension in rats (Farzaei et al.
2016b). Adjunctive therapy of curcumin (500 mg/day for 4 weeks)
with anti-helicobacter regimen ameliorated the symptoms of
dyspepsia in peptic ulcer patients (Khonche et al. 2016). In
animal study, curcumin administration reduced the restraint
stress and water immersion stress-induced gastric lesions by
increasing gastric blood flow and attenuating pentagastrin or
histamine-stimulated secretion of gastric acid. In addition, the
expression levels of iNOS, COX-2 and TNF-awas significantly
downregulated in gastric mucosa of curcumin administered rats
exposed to restraint stress and water immersion stress,
resulting in gastroprotective effect (Czekaj et al. 2016).
Curcumin (10, 50 or 100 mg/kg orally for three days) dose
dependently reduced LPO and gastric ulcer area and restored GPx,
CAT and SOD levels in gastric mucosa of naproxen treated rats
(Kim et al. 2016b). Curcumin treatment reversed stress mediated
gastric ulceration in rats by reducing the hemorrhage of gastric
mucosa, increasing gastric pH values and attenuating ulcer index
which is associated with downregulation of histone H3
acetylation at H þ , K þ -ATPase promoter gene (He et al.
2015a). Curcumin treatment decreased pepsin activity, total acid
output and ulcer index alongside reduced MDA level, ameliorated
mucin, CAT, NO and SOD in gastric mucosa of indomethacin-induced
ulcer in rats (Morsy and El-Moselhy 2013). Additionally,
curcumin ameliorated indomethacin-induced gastric ulcer by
inducing angiogenesis and collagenization of gastric tissue via
upregulation of TGF-b, MMP-2, membrane type 1-MMP and VEGF
expressions in ulcerated tissues (Sharma et al. 2012). The
biological mechanism of curcumin to combat peptic ulcer is
mainly due to its antioxidant and anti-inflammatory activities.
The gastroprotective effect is also due to inhibition of acid
release, amelioration of blood flow, angiogenesis and
collagenization of gastric tissue (Sharma et al. 2012; Yadav et
al. 2013). Clinically, curcumin administration (40 mg orally,
three times a day, for four weeks) reduced the production of
IL-1b, IL-8, COX-2 and TNF-ain gastric mucosa, and attenuated
inflammation in gastritis patients infected with H. pylori
bacteria (Koosirirat et al. 2010). In a randomized clinical
trial, administration of curcumin (700 mg orally, three times a
day, for 4 weeks) reduced the level of MDA, DNA oxidative
damage, endoscopic and chronic inflammation scores and glutathione peroxides in gastritis patients (Judaki et al.
2017). In animal model, curcumin treatment downregulated the
expression of chemokines such as CXCL1, CCL5, CXCL10, CXCL11,
CCL20 and Chemokine (C-C motif) ligand 25 in stomach of mice
bearing Helicobacter pylori induced gastric inflammation. In
addition, curcumin decreased secretion of IL-1b, IL-6 and
TNF-aduring H. pylori infection. Further, curcumin
supplementation reduced the macromolecular leakage, MyD88
expression and NF-jB activation in gastric cells (Santos et al.
2015). Study suggested that antimicrobial activity of curcumin
against H. pylori is responsible for the management of gastritis
in mice (De et al. 2009). The biological effect of dietary
polyphenol curcumin to reduce chronic gastritis is mainly due to
its antioxidant, anti-inflammatory and anti-bacterial activities
(Yadav et al. 2013), therefore, it can be recommended as a novel
drug for management of gastritis. One open-label study evaluated
the efficacy of curcumin in five patients with ulcerative
proctitis and in five patients with Crohn disease.
Significant decrease in symptoms as well as in inflammatory
indices (erythrocyte sedimentation rate and CRP) were observed
in all patients with proctitis This study suggests the efficacy
of curcumin against IBD. Another study evaluated the efficacy of
curcumin as maintenance therapy in 89 patients with quiescent
ulcerative colitis. The relapse rates were 4.65% in the
curcumin-treated group and 20.51% in the placebo group. In
another recent study, ingestion of oral curcumin at 500 mg/day
along with prednisone was associated with clinical and
endoscopic remission in a 60-year-old woman with a 17-year
history of left-sided ulcerative colitis and enteropathic
arthropathy (34). The patient had been examined for persistently
active colitis in December 2009. Both a clinical and endoscopic
evaluation confirmed the diagnosis. Previously, multiple
mesalamine preparations, sulfasalazine, and steroid enemas had
not been effective, and the patient had required multiple
courses of steroids for disease exacerbation. She refused
azathioprine/6-mercaptopurine and anti-TNF treatment because of
possible adverse effects. In addition to 40 mg of prednisone,
500 mg of curcumin per day was given to the patient. After
receiving curcumin and prednisone treatment for 1 year, the
patient’s bowel movements had gone to two per day without blood,
she was no longer taking steroids, and she was feeling well. She
remained in clinical remission at further clinical evaluations
in April, July, and December 2010. A colonoscopy performed in
September 2010 showed no ulceration and biopsies consistent with
chronic inactive ulcerative colitis (34). Thus, based on this
case study, curcumin represents a viable treatment alternative
or adjunctive therapy in the management of chronic ulcerative
colitis. A recent study assessed the effect of curcumin on the
levels of p38 mitogen-activated protein kinase (p38 MAPK),
IL-1β, IL-10, and matrix metalloproteinase-3 (MMP-3) in the gut
of children and adults with IBD. Results indicated suppression
in p38 MAPK activation, reduction in IL-1β, and enhancement in
IL-10 levels in curcumin-treated mucosal biopsies. Furthermore,
dose-dependent suppression of MMP-3 in colonic myofibroblasts
was observed after curcumin treatment. Another study conducted
with eight healthy participants reported that turmeric has the
potential to increase bowel motility and to activate
hydrogen-producing bacterial flora in the colon. |
|
How may Curcumin work against
metabolic diseases such as polycystic ovary syndrome, metabolic
syndrome, and obesity? |
Effect of Curcumin on Metabolic Syndrome
| The role of curcumin in reducing oxidative stress and
inflammation has far-reaching implications when it comes to
overall metabolic health. The idea that curcumin can attenuate
systemic inflammation has implications for metabolic syndrome
(MetS), which includes insulin resistance, hyperglycemia,
hypertension, low high-density lipoprotein cholesterol (HDL-C),
elevated low-density lipoprotein cholesterol (LDL-C), elevated
triglyceride levels, and obesity, especially visceral obesity.
Curcumin has been shown to attenuate several aspects of
metabolic syndrome by improving insulin sensitivity, suppressing
adipogenesis, and reducing elevated blood pressure,
inflammation, and oxidative stress. In addition, there is
evidence that curcuminoids modulate the expression of genes and
the activity of enzymes involved in lipoprotein metabolism that
lead to a reduction in plasma triglycerides and cholesterol and
elevate HDL-C concentrations. Curcumin has been shown to have a
role in decreasing angiogenesis and adipogenesis by suppressing
CCAAT/enhancer-binding protein alpha and PPAR expression and by
lowering cholesterol levels. Moreover, curcumin has the ability
to upregulate the gene expression of pancreatic glucose
transporter 2 (GLUT2), GLUT3, and GLUT4, thus stimulating
insulin secretion. In a randomized double-blind
placebo-controlled trial with a parallel-group design, 117
subjects with metabolic syndrome received either 1 g curcumin
plus 10 mg piperine to increase absorption or a placebo.
Analysis revealed significant reductions in serum concentrations
of TNF-α, IL-6, transforming growth factor beta (TGF-b), and
monocyte chemoattractant protein-1 ( MCP-1) following curcumin
piperine supplementation. The results of this study suggest that
curcumin piperine supplementation significantly decreases serum
concentrations of pro-inflammatory cytokines in subjects with
metabolic syndrome. In addition, the study looked at the
cholesterol-lowering properties and found that curcuminoids were
more effective than the placebo in reducing serum LDL-C,
non-HDL-C, total cholesterol, triglycerides, and lipoprotein a
(Lp(a)), in addition to elevating HDL-C concentrations. The
effects of curcuminoids on triglycerides, non-HDL-C, total
cholesterol, and Lp(a) remained significant after adjustment for
baseline values of lipids and body mass index. From the same
study, the authors also reported markers of oxidative stress.
There was a significant improvement in serum SOD activities (p <
0.001) and reduced MDA (p < 0.001) and C-reactive protein (CRP)
(p < 0.001) concentrations in the group receiving the curcumin
with piperine compared to the placebo group. Quantitative data
synthesis revealed a significant effect of curcuminoids vs.
placebo in reducing circulating CRP concentrations. The authors
concluded that short-term supplementation with a
curcuminoid-piperine combination significantly improves
oxidative and inflammatory status in patients with metabolic
syndrome. Curcuminoids with piperine could therefore be regarded
as natural, safe, and effective CRP-lowering agents.
Inflammatory cytokines were also measured in the above study.
Mean serum IL-1β (p = 0.042), IL-4 (p = 0.008), and vascular
endothelial growth factor (VEGF) (p = 0.01) were found to be
significantly reduced by curcumin piperine therapy. The authors
suggest that the findings indicate that curcumin piperine may
exert immunomodulatory effects via altering the circulating
concentrations of IL-1β, IL-4, and VEGF. In a randomized
double-blind placebo-controlled crossover trial, 36 obese adults
received either 1 g curcumin and 10 mg piperine or a placebo for
30 days followed by a two-week washout period, after which they
received the other treatment. A significant reduction in serum
triglyceride concentrations was observed.
Effect of
Curcumin on Obesity |
Obesity is Inflammatory Disease.
Studies have suggested that properties in curcumin may have a positive effect on blood sugar
and blood pressure and may also promote weight loss and prevent
obesity. Scientists led by Dr David
Fairlie from the University of Queensland, Australia, have found
abnormal amounts of an inflammatory protein called PAR2 in the
fat tissues of overweight and obese rats and humans. PAR2 is
also increased on the surfaces of human immune cells by common
fatty acids in the diet. When obese rats on a diet high in sugar
and fat were given a new oral drug that binds to PAR2, the
inflammation-causing properties of this protein were blocked, as
were other effects of the high-fat and high-sugar diet,
including obesity itself. In the prevention and treatment of obesity and metabolic
syndrome, Curcumin has been reported to modulate numerous targets that have been
linked to obesity and insulin resistance. 1) Curcumin has been shown to downregulate the expression of TNF in various tissues.
2) Curcumin can suppress NF-κB activation induced
by a wide variety of inflammatory agents through inhibition of degradation of
IκBα. 3) Curcumin can inhibit the activation of IKK linked to the activation
of NF-κB, and this leads to the suppression of expression of inflammatory
biomarkers such as cyclooxygenase-2 (COX-2) and vascular endothelial growth
factor. 4) Curcumin has been shown to downregulate the expression of
various NF-κB-regulated proinflammatory adipocytokines including chemokines
(such as MCP-1, MCP-4, and eotaxin)
and interleukins (IL-1, IL-6, and IL-8). Curcumin also suppressed the expression
of plasminogen activator inhibitor type-1 through the inhibition of the
transcription factor early growth response (Egr)-1 gene product that has been
closely linked with insulin resistance and obesity. 5) Curcumin has been reported
to mimic most antidiabetic drugs in that it activates PPAR-γ in hepatic stellate
cells. 6) Curcumin has been shown to downregulate activation of
c-Jun NH2 terminal kinase. 7) Curcumin has been shown to inhibit the Wnt/β-catenin
pathway, which is closely linked to obesity. Later studies have indicated that
Curcumin inhibits Wnt pathway signaling through downregulation of the
transcription coactivator p300. Another potential mechanism by which Curcumin
could inhibit β-catenin signaling is through inhibition of glycogen synthase
kinase (GSK)-3β, which directly causes the phosphorylation of β-catenin.
Curcumin was found to inhibit GSK-3β with as little as 66 nM IC50 (32).
8) Curcumin has been shown to induce the expression of hemeoxygenase (HO)-1
through the activation of Nrf2 in pancreatic cells and thus mediate the survival
of these cells. 9) Curcumin downregulates the secretion of insulin-like growth
factor-1 but induces the expression of insulin-like growth factor binding
protein-3. 10) Curcumin interrupts leptin signaling
by reducing phosphorylation levels of the leptin receptor (Ob-R) and its
downstream targets. 11) Curcumin suppresses gene expression of Ob-R in
HSCs. 12) Curcumin has been reported to increase the expression of
adiponectin, which negatively controls obesity. A study conducted by researchers
at
Tufts University found that curcumin, the predominant
polyphenol in turmeric, suppressed the growth of fat tissue in
mice and cell models and ultimately reduce weight gain. Two
groups of mice were fed high-fat diets—one supplemented with 500
mg of curcumin per kilogram of weight. The curcumin group did
not gain weight as the high-fat-only group. Clinically,
chronic administration of curcuminoids (comprising curcumin,
bisdemethoxycurcumin and demethoxycurcumin) significantly
decreased serum pro-oxidant-antioxidant balance, oxidative
stress burden (Sahebkar et al. 2013), serum triglycerides
(Mohammadi et al. 2013), VEGF, IL-1band IL-4 in obese patients
(Ganjali et al. 2014). In animal study, curcumin treatment
reduced the level of triglyceride and LDL-cholesterol along-
side increased HDL-cholesterol, which is known to ameliorate
lipoprotein metabolism. Curcumin administration (0.05% w/w of
diet) markedly decreased the plasma level of free fatty acid and
triglyceride in the hamsters fed with high-fat diet (10% coconut
oil and 0.2% cholesterol w/w) (Ganjali et al. 2017). Curcumin
administration (200 mg/kg, dissolved in 0.1% carboxy methyl
cellulose, for 10 weeks) significantly decreased body weight,
adipose weight, liver weight, plasma levels of triacylglycerol,
lipid ratios, hepatic fat accumulation while increased HDL in
fructose-fed rats (Maithilikarpagaselvi et al. 2016). Curcumin
administration alone (80 mg/kg/day, p.o., for 12 weeks)
significantly down-regulated the hepatic expression of sterol
regulatory element-binding proteins-1, sterol regulatory
element-binding proteins-2, 3-hydroxy-3-methylglutaryl-coenzyme
A reductase, mevalonate kinase, 24-dehydrocholesterol reductase,
7-dehydrocholesterol reductase, lanosterol synthase, sterol-
C4-methyl oxidase-like (Sc4mol), squalene synthase, proprotein
convertase subtilisin/kexin type 9, LDL-receptor, acetyl-
coenzyme A carboxylase-1, ATP citrate lyase, acyl-CoA syn-
thetase, fatty acid synthase, fatty acid desaturase-1, fatty
acid desaturase-2, stearoyl-coenzyme A desaturase-1, glycerol-3-
phosphate acyltransferase, glucose-6-phosphatase and phos-
phoenolpyruvate carboxykinase-1 in high fat diet-induced obese
mice. In addition, curcumin administration upregulated the
hepatic phosphorylation of IRS-1, IRS-2 and Akt at serine 473
resulting in reversal of obesity in mice (Ding et al. 2016).
Curcumin administration (200 mg/kg body weight) with high fat
diet for 10 weeks significantly decreased the hepatic ERK and
p38 signaling pathway activation as well as reduced body weight
in rats (Maithili Karpaga Selvi et al. 2015). Curcumin (1 g/kg)
along with high fat diet containing 60% of total calories from
fat (5.1 kcal/g diet) administration for 16 weeks significantly
decreased hepatic lipids levels, lipid peroxidation. Curcumin
(100 or 400 mg/kg) along with high fat diet for 8 weeks
effectively reduced serum fetuin-A levels and hepatic
triglycerides level in obese rats. Curcumin is known to inhibit
NF-jB activation and macrophage infiltration in adipose
tissue. In addition, curcumin downregulated the expression of
the plasminogen activator inhibitor type-1, TNF-aand MCP-1 while
upregulated the expression of adiponectin in adipocytes
(Bradford 2013). In in vitro assay, curcumin downregulated the
expression of axin, GSK-3b, CK1-a, AP-2 (mature adipocyte
marker) and upregulated the expression of Fz2 (Wnt direct
receptor), Wnt10b, LRP5 (Wnt co-receptor), c-Myc and cyclin D1
in 3T3-L1 cells. In addition, curcumin inhibited the
phosphorylation of MAPK, JNK, p38 and ERK thereby rescue the
differentiation of 3T3-L1 cells into adipocytes (Ahn et al.
2010). Curcumin treatment inhibited mitotic clonal expansion
process and downregulated the expression of PPAR-c, kruppel-like
factor 5 and C/EBParesulting in reduced adipocyte
differentiation (Kim et al. 2011). Mechanistically, curcumin
administration inhibits NF-jB activation and macrophage
infiltration, reduces the expression of plasminogen
activator inhibitor type-1, MCP-1, TNFa, very low density
lipoprotein (VLDL), cytokines and leptin alongside induced HO-1,
fatty acid oxidation, APO-A1 and adiponectin level. In addition,
curcumin treatment reduces the incidence of obesity and its
associated risk factors, mainly due to its antioxidant and
anti-inflammatory activities (Alappat and Awad 2010).
Effect of Curcumin on Polycystic Ovary Syndrome (PCOS)
| The latest systematic review and meta-analysis of
randomized-control trials investigated a significant improvement
in fasting glucose, fasting insulin, the homeostasis model
assessment measuring insulin resistance (HOMA-IR), and the
quantitative insulin sensitivity check index (QUICKI) in women
with PCOS who took curcumin in comparison with a placebo group.
Jamilian et al. found that administration of curcumin for 12
weeks in women with PCOS had beneficial effects on glycemic
control, among other things. The researchers reported that
reduced fasting glucose (p = 0.002) significantly increased
insulin sensitivity (p = 0.02), and positive alterations in
serum lipids (i.e., a decrease in total cholesterol (p = 0.001)
and LDL cholesterol (p = 0.001) and an increase in HDL
cholesterol levels (p = 0.01)) in comparison to patients taking
a placebo; in addition, curcumin supplements decreased the
weight of women suffering from PCOS. Other researchers who have
looked at the effects of curcumin on glycemic status, lipid
profile, and high-sensitivity C-reactive protein (hs-CRP) levels
in overweight/obese women with PCOS found that serum insulin,
QUICKI (p < 0.05), and HOMA-IR (p = 0.067) were significantly
improved in the group treated with curcumin. In contrast, the
differences in lipid parameters and hs-CRP levels were not
statistically significant in the curcumin-treated group.
Curcumin may stimulate insulin-mediated glucose uptake through
the phosphatidylinositol 3-kinsase (PI3K)/Akt pathway, which, in
turn, upregulates glucose transporter 4 (GLUT4) in the adipocyte
and skeletal muscle, leading to an increase in glucose levels.
Additionally, curcumin may also enhance GLUT4 and glucose uptake
in adipocytes. Curcumin has been shown to inhibit liver
gluconeogenesis through modulation of 5’AMP-activated protein
kinase (AMPK), thus reducing blood glucose levels. Moreover, the
lipid-lowering potential of curcumin may be a consequence of
curcumin’s ability to decrease the circulatory levels of lipid
peroxides and total serum cholesterol (TC), or to increase the
levels of high-density lipoprotein (HDL). There are some
potential mechanisms that may be responsible for the beneficial
influence of curcumin on lipid profile; for example, curcumin
may suppress the expression of Niemen-Pick C1-like (NPC1)
protein in the intestine, which mediates the cholesterol
absorption of hepatocytes. Curcumin also ameliorates
dyslipidemia and activates the lipid metabolism pathway, which
elevates lipoprotein lipase activity to decrease triglyceride
levels. The hypothesis about the beneficial impact of curcumin
supplementation on women with PCOS is because curcumin may
support the improvement in complications of PCOS by regulating
gene expression—that is, by increasing the gene expression of
superoxide dismutase (SOD) and glutathione peroxidase enzymes
(GPx)—and cellular signaling. One of the first studies on the
effects of curcumin on postprandial glucose and insulin
response, which was conducted in 2010 by Wickenberg et al.
showed the possible effects of curcumin on postprandial insulin
levels. Curcumin is generally assumed to improve the body’s
antioxidant enzymes by impacting related gene expression in
patients with PCOS. In a randomized and double-blinded clinical
trial involving 67 overweight or obese female patients with
PCOS, the effects of curcumin on gene expression of peroxisome
proliferator-activated receptor γ coactivator-1α (PGC-1α) (p =
0.011) and silent information regulator 1 (SIRT1) were reported.
SIRT1 contributes to the deacetylation of the PGC-1α gene,
thereby increasing the rate of thermogenesis and oxidation of
lipids, and is also an NAD+-dependent histone deacetylase in the
pathway of insulin secretion [15]. These results would seem to
suggest that curcumin may improve hormonal profiles in patients
with PCOS due to its support of ovarian function by reducing
inflammation and oxidative stress. Interestingly, Sohrevardi et
al. reported that the hormonal parameter of total testosterone
levels and the biochemical parameters of triglycerides, HDL, and
total cholesterol were significantly improved in the group
taking curcumin together with metformin after three months in
comparison to the group treated with only metformin. The
anti-inflammatory properties of curcumin may mitigate
hyperandrogenism due to its possible role in glucose and lipid
metabolism. Moreover, curcumin has the ability to lower
circulating tumor necrosis factor alpha (TNF-α) and interleukin
6 (IL-6) concentration. Proinflammatory cytokines such as TNF-α
have been found to be significantly higher in PCOS patients
[25]. Moreover, curcumin may exert anti-diabetic effects by
increasing the gene expression of PPAR-γ, which has a
pleiotropic impact on glucose homeostasis and insulin
sensitivity and controls gene expression in lipid and glucose
metabolism. Heshmati et al. [28] reported not only reduced
glucose and insulin plasma levels, but also significantly
reduced serum dehydroepiandrosterone (DHEA) levels (−26.53
μg/dL; p = 0.035) in patients with PCOS who took curcumin
supplements for 12 weeks in doses of 1500 mg per day in
comparison to the placebo patients. To date, there are few
studies that have assessed the influence of curcumin on the sex
hormones in women with PCOS. Regarding proinflammatory
cytokines, Mohammadi et al. [31] investigated the therapeutic
effects of curcumin on TNF-α, IL-6, and C-reactive protein (CRP)
in rats with PCOS. The difference between the curcumin-treated
group and the non-curcumin-treated rats with PCOS was
significant. The results showed decreased IL-6 and CRP, and
interestingly, they observed decreased expression of tumor
necrosis factor alpha (TNF-α) in the granulosa layer and
follicular fluid of follicles and ovarian cysts in the PCOS
group treated with curcumin. |
| How may Curcumin work
against liver diseaseS such as Non-alcoholic fatty liver disease
(NAFLD) or metabolic-associated fatty liver disease (MAFLD), cirrhosis,
and hepatitis? |
Effect of
Curcumin on Non-Alcoholic Fatty Liver Disease (NAFLD) or
Metabolic-Associated Fatty Liver Disease (MAFLD)
|
Curcumin and related phenolics have been linked with the
inhibition of lipid peroxidation, free radical formation (e.g.,
neutralization of superoxide, peroxyl, and hydroxyl radicals
(ROSs), nitric oxide, and peroxynitrite (RNS)) and DNA damage.
Despite obesity and hyperlipidemia, it is also known that
patients with type 2 diabetes have a high prevalence of NAFLD
(up to 70%). The above diseases share multiple cardiometabolic
risk factors and proinflammatory pathways. Różański et al.
analyzed databases and publications that have described the
effects of using curcumin supplementation on biochemical
parameters in MAFLD. They concluded that curcumin may have
therapeutic potential in MAFLD patients. Jalali et al. included
nine relevant randomized controlled trials (RCTs) in their
meta-analysis in order to study the effects of curcumin
supplements on metabolic markers and anthropometric parameters
in patients with NAFLD. As shown in Table 3, the study reported
a significant decrease not only in alanine transaminase (ALT) (p
= 0.049) and aspartate transaminase (AST) (p = 0.032) activity,
but also in serum total cholesterol (TC), LDL, FBS (p = 0.027),
HOMA-IR (p = 0.031), serum insulin, and waist circumference
(WC). After a meta-regression analysis of the duration and a
dosage-based analysis, a significant change in BMI was
indicated, and a subgroup analysis (age-based and TC-based) also
indicated a significant decrease in TG. The study investigated
changes in two-month and three-month supplementation with
curcumin. The authors concluded that the use of curcumin in the
analyzed studies had a beneficial effect on both metabolic and
anthropometric parameters in patients with NAFLD. Curcumin administration (60
mg/kg for 4 weeks) inhibited the bio- synthesis of unsaturated
fatty acids and fatty acids synthesis in ethanol treated mice.
In addition, ethanol induced hepatic steatosis was reversed by
curcumin treatment (Guo et al. 2017). Animal studies have shown
that curcumin administration reduced the ethanol-induced
increase in MDA content, decreases the levels of aspartate
aminotransferase (AST) and lactate dehydrogenase (LDH), and
increases the GSH levels. In addition, it is known to reduce
fatty liver, oxidative stress, inflammation and necrosis (Nabavi
et al. 2014; Nanji et al. 1999; Ghorbani, Hajizadeh, and
Hekmatdoost 2016). Non-alcoholic fatty liver disease is an
umbrella term for a variety of pathological conditions including
steatosis, fibrosis, cirrhosis and steatohepatitis, caused by
accumulation of fat in the liver. It is closely correlated with
metabolic syndrome, obesity, overweight and type 2 diabetes in
pediatric and adult individuals (Nabavi et al. 2014). In
randomized placebo-controlled trial, curcumin administration (70
mg/day for two months) significantly reduced the liver fat
content, triglycerides, LDL-cholesterol, serum levels of total
cholesterol, body mass index, ALT, AST, glycated hemoglobin and
glucose in patients with nonalcoholic fatty liver disease as
compared to placebo group (Rahmani et al. 2016). Additionally,
curcumin upregulated the expression of adiponectin precursor and
reduced its methylation in experimental model of fatty liver
disease (Park et al. 2016). In methionine and choline feed
deficient mouse model, curcumin administration inhibited the
activation of NF-kB and reduced the inflammatory recruitment in
steatohepatitis (Leclercq et al. 2004). Curcumin administration
downregulated the intrahepatic expression of procollagen type
I, CD11b, tissue inhibitor of metalloprotease (TIMP)-1, monocyte chemoattractant protein-1 and a-smooth muscle-actin in
methionine and choline feed deficient mouse model of
steatohepatitis alongside reduced the oxidative stress in
cultured stellate cells (Vizzutti et al. 2010). Curcumin
administration reduced the serum hepatic markers viz., AST, ALT
and MDA thereby attenuated lipopolysaccharide/d-galactosamine
induced liver damage in rats. In the same study, curcumin
administration reduced the NF-jB activation and TNF-a level in
liver and serum. Furthermore, curcumin upregulated
Nrf-2-dependent antioxidant defense genes like quinone (NQO-1),
NAD(P)H dehydrogenase, glutamate-cysteine ligase and heme
oxygenase-1 which is responsible for the hepatoprotective
activity (Xie et al. 2017). Curcumin administration ameliorated
the barrier integrity of intestine, reduced ectopic fat
deposition in liver and modulated the gut microbiota which in
turn reversed hepatic steatosis in high fat diet fed rats (Feng
et al. 2017). Curcumin administration elicited hepatoprotective
effect via reversal of reduced GPx, CAT and SOD levels in
tartrazine induced liver injury. In addition, it reduced the
intracellular vacuolization, dilation of central vein and
sinusoids as well as necrosis in hepatotoxic rats (El-Desoky et
al. 2017). Recent experimental evidence suggests that curcumin
administration reduced Gr1hi monocytes infiltration in liver,
downregulated the expression of MCP-1, TNF-aand TGF-b1 in mouse
model of CCl 4 induced liver fibrosis (Huang et al. 2016b).
Effect of Curcumin on Cirrhosis | It was reported that curcumin
administration prevents bile duct ligation induced cirrhosis in
rats via inhibition of oxidative stress and downregulation of
TGF-b(Reyes-Gordillo et al. 2008). Curcumin administration ameliorated the functional properties of
hepatocytes and downregulated the expression of NF-jB and iNOS
in liver of biliary duct ligated rats (Barta et al. 2015).
Effect of Curcumin on Hepatotoxic Ailments
| Curcumin is said to increase apoptosis in injured hepatocytes
while also reducing inflammatory effects, hepatic fibrogenesis,
and substantially liver injury. The hepatoprotective attribute
of curcumin might be due to direct free radical scavenging
mechanisms, boosting glutathione levels, and assisting in liver
detoxification. Curcumin has hepatoprotective activity similar
to that of silymarin. From studies, it can be concluded that
curcumin has hepatoprotective potential in various
including carbon tetrachloride (CCl4), acetaminophen (paracetamol)
and galactosamine. This hepatoprotective effect is mainly a
observed due to the antioxidant activity of curcumin along with
its ability to decrease the formation of proinflammatory
cytokines. Administration of curcumin is resulted in decrease of
liver injury. Aflatoxin-induced biliary hyperplasia, lipid
alterations, and necrosis were likewise cured by curcumin.
Sodium curcuminate is a salt of curcumin that has choleretic
effects, boosting biliary excretion of bile salts, cholesterol,
bilirubin, and bile solubility, thus helping to prevent and
treat cholelithiasis. This could be related to the antioxidant
capacity of curcumin’s phenolic groups. Tacrine is well-known
for its hepatotoxic and T-cell-destructive properties. Curcumin
was over ten times more efficient than standard therapy,
ascorbic acid, in research involving human hepatocytes cells
that had been disrupted by tacrine (Song et al., 2001).
Effect of Curcumin on Hepatitis | A recent
in vitro study demonstrated that curcumin treatment time and
dose dependently reduce the expressions of hepatitis B virus
surface antigen and e-antigen in hepatitis B virus transfected
HepG2.2.15 cell line. In addition, curcumin inhibited
replication of hepatitis B virus gene via down-regulation of
cccDNA-bound histone acetylation (Wei et al. 2017). Study
revealed that curcumin treatment inhibits hepatitis B virus via
downregulation of the metabolic coactivator peroxisome
proliferator-activated receptor gamma coactivator 1-alpha
(PGC-1a). It has been reported that combination of
nucleotide/nucleoside analog with curcumin can synergistically
reduce the replication of hepatitis B virus (Nabavi et al. 2014;
Mouler Rechtman et al. 2010). It was reported
that co-incubation of hepatitis C virus with curcumin and its derivatives
potently inhibits the entry of all major hepatitis C virus genotypes. Curcumin affects the membrane fluidity
resulting in impairment of viral binding and fusion thereby
inhibits cell-to-cell transmission in human liver cells
(Colpitts et al. 2014). Co-administration of curcumin and
IFN-a profoundly inhibited hepatitis C virus replication in Huh7 cells and
found to be effective against hepatitis C virus infections (Kim et al. 2010).
Moreover, curcumin exhibited anti-HCV activity by inducing HO-1
and modulating ERK and NF-jB activities in Huh7.5 cells
expressing the hepatitis C virus genotype 1 b subgenomic replicon (Chen et al.
2012). Mechanistically, curcumin shows hepatoprotective action
due to its antioxidant effects and inhibitory activity against
NF-jB that is known to regulate different pro-fibrotic and
pro-inflammatory cytokines. Additionally, curcumin supple-
mentation reduced liver marker enzymes, cholesterol levels and
replication of hepatitis B and C viruses (Nabavi et al. 2014). |
| How may Curcumin work as
an antimicrobial, Antibacterial, Antiviral, antiparasitic, and Antifungal? |
Curcumin has antiviral potential (von
Rhein et al., 2016) even for HIV; inhibiting HIV-1 LTR promoter
directed gene expression with no effect on cell viability
(Ashraf, 2018). Curcumin had moderate effectiveness towards
Plasmodium falciparum and Leishmania organisms.
The ethanol extracts exhibit anti-Entamoeba histolytica
activity while curcumin has anti-P. falciparum and
anti-Leishmania effect in vitro. Curcumin seems to have
its antiviral activity for Epstein–Barr virus and HIV (Taher et
al., 2003). An extract of C. longa in both aqueous and ethanol
is used in aquaculture as a treatment for bacterial infections (Sahu
et al., 2005). Curcumin exerts anti-parasitic action against
African trypanosomes, has schistosomicidal activities against
Schistosoma mansoni adult worms, and has anti-malarial
in addition to nematocidal effects. Curcumin has shown a wide
range of antiviral activity against different viral models.
Similar to these reports, our findings indicated that curcumin
inhibits SARS-CoV-2 D614G strain which contains the most
widespread amino acid change (D614G in the spike protein)
carried by more than 99% of the prevalent variants since the
beginning of 2020. According to previous reports, curcumin
exhibited moderate selectivity for pre-infection and
post-infection treatment strategies. Contrarily, low selectivity
was obtained for pre–post infection treatment and co-treatment
strategies. Curcumin inhibited SARS-CoV-2 D614G strain by
pre-infection treatment of Vero E6 cells. This effect has also
been observed with other enveloped viruses such as Influenza,
Dengue, Zika, Chikungunya, Japanese encephalitis, Pseudorabies,
and Vesicular stomatitis virus, showing that curcumin treatment
affects the early stages of the replicative cycle, such as viral
attachment, internalization, fusion, or decapsidation. With
regard to SARS-CoV-2, spike protein binds to its human receptor
ACE2 (angiotensin-converting enzyme 2) through its
receptor-binding domain. Previous studies have reported a
favorable binding affinity of curcumin to the spike protein and
its cell receptor, ACE2 (angiotensin-converting enzyme).
According to the above, it could be suggested that curcumin
prevents the recognition of the target cell and subsequent
SARS-CoV-2 entry by direct interaction with cell factors or
viral proteins. This effect could be related to our results
obtained by co-treatment which suggest a possible virucidal
activity of curcumin against SARS-CoV-2 D614G strain. It has been demonstrated
that curcumin as a plant derivative has a wide range of
antiviral activity against a variety of viruses including
parainfluenza virus type 3 (PIV-3), feline infectious
peritonitis virus (FIPV), vesicular stomatitis virus (VSV),
herpes simplex virus (HSV), flock house virus (FHV), and
respiratory syncytial virus (RSV) assessed by MTT test showed
the potent antiviral activity of curcumin and its bioconjugates
against different viral pathogens for further studies. Curcumin
showed the anti-influenza activity against influenza viruses
PR8, H1N1, and H6N1. The results showed more than 90% reduction
in virus yield in cell culture using 30 μM of curcumin. In H1N1
and also H6N1 subtypes, the inhibition of haemagglutinin
interaction reflected the direct effect of curcumin on
infectivity of viral particles and this has proved by time of
drug addiction experiment. Additionally, unlike amantadine,
viruses developed no resistance to curcumin. There are
several studies illustrate that curcumin can impede viral
replication and prevent injuries caused by several virus
infectious diseases in particular, for RNA virus infections. In
vitro and in vivo results have shown that curcumin effectively
moderates infections and symptoms caused by the hepatitis virus,
respiratory syncytial virus (RSV), Human Immunodeficiency Virus
(HIV/AIDS), Zika virus, Chikungunya virus, Epstein Barr
virus, papilloma virus (HPV), enterovirus (enterovirus),
Japanese encephalitis virus, influenza virus, dengue virus, and
coronavirus (such as SARS-COVID 19) In recent studies that have
calculated and simulated molecular docking models for viral
infections, the results showed that the curcumin molecule can
directly interact with proteins of Ebola virus, influenza virus,
AIDS Viruses, dengue virus, and human papillomavirus (HPV), etc.
The combination of curcumin with viral coat proteins,
virus-specific enzymes, or RNA polymerase can affect and abolish
virus replication, infection, and damage to cells. Current
research results show that turmeric can inhibit SARS and COVID
19 infections via molecular binding, and currently curcumin is
also applied in clinical trial on COVID 19 infection. Antimicrobial activities for curcumin and rhizome
extract of C. longa against different bacteria, viruses,
fungi, and parasites have been reported. The promising results
for antimicrobial activity of curcumin made it a good candidate
to enhance the inhibitory effect of existing antimicrobial
agents. Curcumin has shown antibacterial activity effectively
against Staphylococcus aureus, Salmonella paratyphi,
Trichophyton gypseum, and Mycobacterium tuberculosis. The
antibacterial activity of the aqueous extracts from turmeric is
believed to be due to the anionic constituents like nitrate,
sulphates, chlorides, and thiocyanate. Antibacterial activity of curcumin was also studied
in endodontic bacteria Streptococcus mutans, Actinomyces
viscosus, Lactobacillus casei, Porphyromonas gingivalis,
Prevotella intermedia, and Enterococcus faecalis, and a
significant inhibition of bacterial growth was observed. Many
studies have revealed that curcumin and turmeric extracts
inhibit the growth of microorganisms. Curcumin has antibacterial
effects on both gram-positive and -negative bacteria, such as:
Staphylococcus aureus, Streptococcus pneumoniae, Salmonella,
Escherichia coli, Helicobacter pylori, etc., which often cause
human infectious diseases. In preclinical and clinical studies
for sepsis treatment, i.e. systemic bacterial infections,
curcumin can act on PI3K/AKT, NFκB, TNF-α and TGF-β1 pathways to
attenuate the toxicity of LPS on sepsis and curcumin also exerts
the protective role in the lungs, liver, and kidneys while
reducing the sequelae of tissue fibrosis after sepsis.
Curcumin is very effective against several pathogenic Gram +ve
bacteria such as Staphylococcus aureus, Staphylococcus
epidermidis and Enterococcus species that cause many infections
such as skin problems, pneumonia, meningitis, and urinary tract
infection. The study of curcumin against 14 strains of Candida
including 4 ATCC strains and 10 clinical isolates showed that
curcumin is a potent fungicide compound against Candida species
with MIC values range from 250 to 2000 μg/mL. In another study,
anti-Candida activity of curcumin was demonstrated against 38
different strains of Candida. A recent study revealed that,
curcumin exhibited in vitro antibacterial activity against most
prevalent organisms like Enterococcus faecalis, Prevotella intermedia, Porphyromonas gingivalis, Actinomyces viscosus,
Lactobacillus casei, Streptococcus mutans and Aggregatibacter
actinomycetemcomitans (Mandroli and Bhat 2013). Moreover,
curcumin demonstrated its effectiveness against
Bacillus
subtilis, Mycobacterium tuberculosis, Escherichia coli,
Helicobacter pylori, Staphylococcus intermedius, Sarcina
lutea, Sarcina lutea and Neiserria gonorrhoeae (Tyagi et al.
2015; Marathe et al. 2011). Curcumin treatment reduced growth of
gut microbiota like Bifidobacterium, E.
faecalis, Bifidobacterium. pseudocatenulatum G4, Bifidobacterium.
longum BB536,E. coli K-12, Lactobacillus acidophilus and
Lactobacillus casei thereby inducing the susceptibility to
infectious disease (Marathe et al. 2011). Curcumin inhibited the
growth of both Gram-negative and Gram-positive bacteria.
Curcumin effectively reduced the infectious disease caused by
various species of Staphylococcus aureus (Tong et al.
2015;Teowetal.2016). Mechanistically, curcumin interfere with
quorum sensing, virulence and biofilm initiation, and inhibits
bacterial cell by suppressing its dynamic assembly. Curcumin
demonstrated its effectiveness against parasites like
Trypanosoma, Plasmodium and Giardia. In parasites culture,
curcumin treatment induced DNA damage via its prooxidant
activity and inhibited histone acetyltransferases in
Plasmodium falciparum resulted in cytotoxicity, which can
be targeted for treatment of malaria (Cui, Miao, and Cui 2007),
revealing its therapeutic potential against cerebral malaria as
adjunctive therapy (Mimche, Taramelli, and Vivas 2011). Curcumin
induced DNA damage and apoptosis and effectively inhibited the
growth of Giardia lamblia (Perez-Arriaga et al. 2006).
Moreover, curcumin administration mediates anti-parasitic
activity against Trypanosoma, a parasite which is
responsible for sleeping sickness and Chagas disease (Marathe et
al. 2011). The biological effect of curcumin to reduce these
infections is mainly due to its pro-oxidant and apoptotic
activities, therefore, it can be recommended as a novel drug for
management of giardia, trypanosoma and plasmodium
infections. Curcumin treatment upregulated the transcription of
chitin synthase-1, chitin synthase-3 and PKC in
Sporothrix
schenckii thus reduced virulence in infected mice (Huang et
al. 2016a). Curcumin induced photodynamic inactivation of the
fungus Candida albicans in murine model of oral
candidiasis (Dovigo et al. 2013). Also, curcumin exhibited
therapeutic potential against oropharyngeal candidiasis in a
mouse model (Karaman et al. 2011). In fungal cell cultures,
curcumin inhibited the growth of wide range of pathogenic fungus
that includes Aspergillus clavatus, Aspergillus terreus,
Aspergillus tamarii, Aspergillus fumigatus, Aspergillus flavus
IMI190443, Aspergillus nomius ATCC 15546, Aspergillus
fumigatus ATCC 16913, Paracoccidioides brasiliensis B339,
Paracoccidioides brasiliensis MG04, Paracoccidioides
brasiliensis 17, Paracoccidioides brasiliensis 608,
Paracoccidioides brasiliensis Pb18, Paracoccidioides
brasiliensis Pb01, Paracoccidioides brasiliensis MG05,
Sporothrix schenckii ATCC 10212, Cryptococcus neoformans ATCC
32608, Candida dubliniensis (Cd28), Candida dubliniensis (Cd22),
Candida glabrata ATCC 2001, Candida parapsilosis ATCC 20019,
Candida krusei ATCC 20298, Candida tropicalis ATCC 750 and
Candida albicans ATCC 18804 (Martins et al. 2008). Curcumin
(500 mg/L) also exhibited antifungal effects against
Phytophthora infestans, Pu. Recondite and Rhizoctonia
solani (Kim, Choi, and Lee 2003). Curcumin demonstrated
fungicidal activity against the clinical isolates of Candida
species like Candida tropicalis, Candida kefyr, Candida
krusei, Candida guilliermondii, Candida glabrata, Candida
parapsilosis and Candida albicans at MIC value of
32–128 mg/mL (Zorofchian Moghadamtousi et al. 2014). The
suggested anti-fungal mechanisms of curcumin includes the
leakage of intracellular component through the flappy membrane,
disruption of fungal plasma membrane, generation of oxidative
stress, induction of early apoptosis, inhibition hyphae
development, upregulation of chitin synthase and PKC etc. (Lee
and Lee 2014; Sharma et al. 2010). These evidences on the
mechanistic action of curcumin could be employed in improving
the treatment strategies for fungal infections. A recent study
has shown that the anti-inflammatory and anti-oxidant effects
conferred by curcumin protect from human cytomegalovirus
infection in Balb/c mice (Lv et al. 2014). Among various
phytochemicals evaluated for antiviral activity against
norovirus, curcumin exhibited most potent anti-noroviral
effects. In a cell culture infection model, curcumin exposure
for 3 days was found to reduce norovirus infectivity by 91%.
Thus, curcumin might be a promising anti-noroviral candidate to
prevent foodborne illness (Yang et al. 2016). Curcumin
demonstrated promising anti-influenza activity against influenza
viruses PR8, H1N1 and H6N1 by interfering with viral
hemagglutination activity (Chen et al. 2010; Dao et al. 2012; Ou
et al. 2013). In dengue infected BHK-21 cells, curcumin
administration reduced the number of plaques produced,
intracellular accumulation of viral proteins and increased the
level of Lys48 ubiquitin-conjugated proteins in dengue virus
(Padilla-S et al. 2014). In in vitro assays, curcumin
demonstrated potent antiviral effect against Human enterovirus
71 (EV71). Curcumin inhibited viral RNA synthesis and expression
of viral protein, thereby decreasing production of viral progeny
(Qin et al. 2014). Proteomics analysis indicated that curcumin
(15–240 lM) pretreatment exert antiviral activity by
downregulating heat shock cognate 71 and inhibited the
replication of viral hemorrhagic septicemia virus (Jeong et al.
2015). On the other hand, curcumin exhibited remarkable
antiviral effects against herpes simplex virus type 1 (HSV-1) by
blocking the recruitment of RNA polymerase II and expression of
viral immediate-early genes (Kutluay et al. 2008). In another
study, curcumin and its metallo derivatives, viz.
gallium-curcumin and Cu-curcumin also exhibited remarkable
anti-HSV-1 activity in vitro (Zandi et al. 2010). Moreover,
curcumin administration conferred significant protection against
intra-vaginal HSV-2 infection (Bourne et al. 1999). Curcumin
inhibited both HIV-1 (IC 50 -100mM) and HIV-2 protease (IC 50
-250mM) thereby suppressed the replication of viral genes and
prevent multiplicity of HIV (Sui et al. 1993). Curcumin mediated
inhibition of HIV protease and integrase (IC 50 40 mM) resulted
in anti-retroviral activity (Mazumder et al. 1997; Mazumder et
al. 1995). Curcumin induced anti-HIV activity can be attributed
to degradation of Tat via proteosomal pathway and inhibition of
Tat protein acetylation by p300/CREB-binding
protein thereby sup- pressed HIV-1 multiplication (Ali and
Banerjea 2016; Balasubramanyam et al. 2004). Curcumin
demonstrated strong anti-HPV activity in cervical and oral
cancer cells through downregulation of HPV oncogene expression
(E6 and E7) of highly oncogenic HPV, HPV-16 and HPV-18 (Divya
and Pillai 2006; Mishra and Das 2015; Prusty and Das 2005).
Curcumin downregulated the transcription factor, AP-1 in HeLa
cells which is critical for transcription of HPV-16 and HPV-18
(Prusty and Das 2005). Curcumin mediated downregulation of viral
oncogenes is attributed to its ability to modulate apoptosis and
prevent NFkB and AP- 1 translocation thereby suppressing the
transcription of HPVs (Divya and Pillai 2006; Prusty and Das
2005). Curcumin exhibited potent antiviral effect against
coxsackie virus by inhibiting viral replication, RNA expression
and protein synthesis via ubiquitin-proteasome system mediated
protein modification or degradation (Si et al. 2005; Si et al.
2007). Mechanistically, curcumin treatment downregulated JunD
protein, reduced production of infective viral particles,
downregulated genomic transcription and translation, inhibited
viral oncoproteins E6 and E7 expressions, suppressed the
Akt/sterol regulatory element-binding proteins (SREBP)- 1
pathway, increased p53 level, inhibited hemagglutination,
inhibited proteases, integrase and Tat protein acetylation
(Zorofchian Moghadamtousi et al. 2014; Mazumder et al. 1995;
Balasubramanyam et al. 2004; Dutta, Ghosh, and Basu 2009). The
extensive research on antiviral activities of curcumin against
different viral pathogens nominates this compound as a potent
antiviral drug candidate.
Effect of Curcumin on
COVID-19 | Curcumin, a natural compound with
anti-inflammatory effect, could as an adjuvant drug in COVID-19
treatment. Curcumin has been revealed to be linked to the viral
S1 protein, which is required for SARS-CoV-2 entry in an in
silico approach; thus, it may inhibit cytokine storm in the
severe stage of COVID-19 (Pawitan, 2020). Curcumin can inhibit SARS-CoV replication. Several studies suggest that curcumin can
inhibit SARS-CoV-2 replication. Evidence has already emerged
regarding the action of curcumin in SARS-associated corona virus
(SARS CO-V) by directly interacting with viral proteins,
disrupting the viral envelope, inhibiting viral proteases and
modulating NFKB, Nrf2 and high mobility group box 1(HMGB1)
pathways in vitro. SARS-CoV-2 (COVID-19) has nearly 79%
resemblance to SARS CO-V and hence the postulation. Curcumin can block the
interaction between the spike glycoprotein and
angiotensin-converting enzyme 2 (ACE2) and inhibit the Nsp15
protein, therefore blocking replication of the virus or
inhibiting viral protease. These observations were supported by
a study by Han et al. who demonstrated that curcumin strongly
inhibited TGEV proliferation and viral protein expression in a
dose and time-dependent manner, and treatment with curcumin
caused a reduction in both viral particles (IC50 of 8.6 μM) and
protein levels in porcine kidney cells. This study suggested
that curcumin may inhibit the adsorption of TGEV or that it
possesses excellent virucidal activity. Compared to the placebo group,
curcumin could reduce the frequency of Th17 cells, Treg and
their related inflammatory factors in both mild and severe
COVID‐19 patients. In addition to anti-inflammatory effect,
curcumin can also play an antiviral role by inhibiting
SARS-CoV-2 entry into cells and inhibiting viral proliferation.
Curcumin has a variety of pharmacological effects and high
safety, which makes it an adjunctive drug for the treatment of
COVID-19. In a clinical trial, orally administered curcumin with
piperine as adjuvant therapy in COVID-19 treatment could
substantially reduce morbidity and mortality, and improve
clinical symptoms. Curcumin effectively neutralized
SARS-CoV-2 at subtoxic concentrations in Vero E6 and human
Calu-3 cells. Furthermore, curcumin treatment significantly
reduced SARS-CoV-2 RNA levels in cell culture supernatants. This
data uncovers curcumin as a promising compound for complementary
COVID-19 treatment. Curcumin concentrations contained in
turmeric root or capsules used as nutritional supplements
completely neutralized SARS-CoV-2 in vitro. Due to the antiviral
as well as anti-inflammatory effect of curcumin, the compound
might have a positive effect on COVID-19 progression. Curcumin
potently neutralizes SARS-CoV-2 in vitro at low subtoxic
concentrations. The good safety profile of curcumin and its
immunomodulatory as well as the antiviral effect make curcumin a
promising candidate for complementary treatment of COVID-19. |
|
How may Curcumin work against depression, major depressive
disorder, and anxiety? |
Curcumin has a wide range of
characteristics that are important to depression pathogenesis.
The extract prevented the decrease in serotonin, noradrenalin,
and dopamine concentrations while increasing serotonin turnover,
cortisol levels, and serum corticotrophin-releasing factor
levels (Xia et al., 2007). The consequences of orally
administered curcumin seem on behavior under chronic stress or
depression condition in the rat model. Curcumin administration
showed a similar impact to imipramine, a known antidepressant
drug, and it has been indicated by various authors to be a
feasible alternative source in depression condition (Mohammed et
al., 2019; Qi et al., 2020). Curcumin has anti-inflammatory,
antioxidant and neurotrophic properties, suggesting it has
strong potential for relieving depression. Curcumin’s
anti-inflammatory effect is one reason for its improvement in
depression. In addition to its anti-inflammatory properties,
curcumin also inhibits the release of monoamine oxidase,
serotonin and dopamine, and regulates the hypothalamus pituitary
adrenal axis, neurotrophic factors, and hippocampal neurogenesis
and neuroplasticity. Administration of curcumin decrease mRNA
expression of proinflammatory cytokines IL-1β, IL-6, and TNF-α,
through down-regulation IL-1β/NF-κB signaling,105 inhibit the
NLRP3 inflammasome activation. Curcumin improves IL-1β-induced
neuronal apoptosis by inhibiting the P38 pathway In a
meta-analysis of nine clinical trials, curcumin may improve
symptoms of depression and anxiety in patients with depression.
In randomized double-blind, placebo-controlled trial, adjuvant
curcumin (doses increased from 500 mg/day to 1500 mg/day) showed
a significant difference between curcumin and placebo at weeks
12 and 16. The core issue of depression has been identified as
inflammation, and
curcumin has been found to be comparable to prescription antidepressant
drugs. In
one study published in
Phytotherapy Research,
scientists studied 60 patients with serious depression over a six-week trial
and found that turmeric was as effective at treating depression as Prozac.
This randomized control trial took 60 volunteers diagnosed
with major depressive disorder and compared the effect of
curcumin to fluoxetine (Prozac). Researchers discovered that the
principal curcuminoid in turmeric is not only as effective as Prozac in
managing depression, but it doesn’t carry with it all the dangerous side
effects as anti-depressive drugs do. One-third of the participants in the
study were given 20 mg of fluoxetine (which is sold under the prescription
names Prozac and Sarafem), one-third were given 1,000 mg of curcumin (the
active ingredient in turmeric), and one one-third were given a combination
of both. "Curcumin, an active ingredient of
Curcuma longa
(Zingiberaceae), has shown potential antidepressant-like activity in animal
studies,” the researchers wrote. “The objectives of this trial were to
compare the efficacy and safety of curcumin with fluoxetine in patients with
Major Depressive Disorder (MDD).” They concluded that curcumin was “well
tolerated” by all the patients. All three groups showed approximately equal
improvement in their depression, whether they were taking the turmeric, the
antidepressant or a combination of both.“This study provides first clinical
evidence that curcumin may be used as an effective and safe modality for
treatment in patients with MDD without concurrent suicidal ideation or other
psychotic disorders. Curcumin, a natural compound derived from the herb
Curcuma longa, exhibits a wide range of pharmacological
properties and thus has been considered as a potent
antidepressant drug. Curcumin may exhibit multiple
antidepressant activities: (a) modulating the neurotransmitter
levels including DA, NE, 5‐HIAA and inhibiting the expression of
monoamine oxidase enzymes; (b) reducing the inflammatory
response by regulating the production of pro‐inflammatory
markers; (c) repairing neurodegeneration and enhancing
neurogenesis and neuronal plasticity typically increased BDNF
levels; (d) improving the activities of antioxidant enzymes; (e)
decreasing the nitric oxide levels; (f) regulating mitochondrial
disturbances; and (g) moderating hypothalamus‐pituitary‐adrenal
(HPA) disturbances. The multiple mechanisms of curcumin provide
a unique advantage in the medication of depression, especially
in the term of adverse effects. A new
study, published online ahead of print in the
Journal of Affective
Disorders, finds that the spice curcumin, a derivative of turmeric, may
be an effective treatment for depression. The study was a randomized,
placebo-controlled trial (the gold standard methodology for medication
studies). The researchers found that curcumin was better than a placebo
treatment, and those with atypical depression were far more likely to
improve. The use of curcumin appeared especially effective for those with
atypical depression. Atypical depression, despite its name, is relatively
common (around 40% of MDD cases). The “atypical” moniker refers to its
particular features: excessive sleep, weight gain, mood improvement in
response to positive events, heavy, immovable feelings in the limbs, and
interpersonal rejection sensitivity. Atypical depression is considered to
have a more chronic course, with worse outcomes overall, so the potential
for a viable treatment with fewer side effects than current medications
provides hope for an improved prognosis. Previous
studies have shown evidence that curcumin could be an
effective
treatment for
depression and found minimal side effects. This study adds to the
literature by comparing several doses of curcumin as well as a
curcumin/saffron combination treatment. A study published in
Brain Research examined the effects of
curcumin administration to laboratory rats after exposure to a chronic
stress protocol. Researchers found that curcumin supplementation had a
beneficial effect on
reducing stress-related symptoms of depression.
A study in Psychopharmacology showed curcumin
increased serotonin production and had an antidepressant effect on
laboratory mice exposed to several lab tests. In a six-week, randomized,
single-blinded, placebo-controlled study in 60 MDD patients, supplemental
curcumin (~880 mg/day of curcuminoids) alone yielded a similar response rate
to the antidepressant, fluoxetine (a serotonin reuptake inhibitor [Prozac];
20 mg/day) in terms of depressive symptoms. A 2009 review published in
Scientific World Journal
hypothesizes that curcumin from turmeric may provide benefits for
depression by
assisting with the regulation of brain neurotransmitters like
dopamine and serotonin and inhibiting the monoamine oxidase enzyme, which
plays a role in breaking down these neurotransmitters. The
neurotransmitters are also what Prozac treats, helping serotonin be used
effectively by the brain. Major depressive
disorder (MDD) is a neuropsychiatric disorder associated with abnormal
neurotransmission; it is primarily treated with drugs that improve the
bioavailability of neurotransmitters like serotonin, noradrenaline, and
dopamine in the brain. Characteristics of MDD also include alterations
in the hypothalamus-pituitary-adrenal axis, increased neuroinflammation,
defective neurogenesis, and neuronal death. A few clinical studies have
examined the effect of curcumin alone or with conventional
antidepressant drugs in MDD patients. A recent meta-analysis of six
randomized controlled trials found that supplementation with curcumin
significantly reduced depression symptoms. Moreover, in a randomized controlled study in 100 participants taking
escitalopram (a serotonin reuptake inhibitor [Lexapro]; 5 to 15 mg/week),
supplemental curcumin (1,000 mg/day) for six weeks increased the
antidepressant effect of the medication. Curcumin also induced a reduction
in plasma concentrations of inflammatory markers and an increase in plasma
concentrations of brain-derived neurotrophic factor compared to placebo
(antidepressant drug alone).
A
study involving 56 people with major depressive disorder
revealed that 500 mg of curcumin taken twice a day for eight
weeks could ease mood-related symptoms. In a controlled
trial, 60 people with depression were randomized into three
groups. One group took Prozac, another group one gram of
curcumin and the third group both Prozac and curcumin. After 6
weeks, curcumin had led to improvements that were similar to
Prozac. The group that took both Prozac and curcumin fared best.
According to this small study, curcumin is as effective as an
antidepressant. Depression is also linked to reduced levels of
brain-derived neurotrophic factor (BDNF) and a shrinking
hippocampus, a brain area with a role in learning and memory.
Curcumin boosts BDNF levels, potentially reversing some of these
changes. There is also some evidence that curcumin can boost the
brain neurotransmitters serotonin and dopamine. A study
published in the journal Acta Poloniae Pharmaceutica found that
curcumin compared favorably to both drugs in reducing depressive
behavior in an animal model. Depression and anxiety are
different neurological disorders, but depressive patients often
experience symptoms like anxiety disorder, such as irritability,
nervousness, and problems in concentrating and sleeping.
Depression and anxiety disorders have its own pathophysiology as
well as behavioral and emotional symptoms. In a double blind,
cross-over clinical trial, curcumin administration (1 g/day for
30 days) significantly reduced anxiety like behavior. Chronic
curcumin administration (500 mg, twice daily for eight weeks) is
associated with elevated urinary level of substance P and
thromboxane B2 as compared to the placebo group. In addition,
curcumin administration ameliorated the plasma endothelin-1 and
leptin which is associated with greater reductions in IDS-SR30,
a major depressive episode (Lopresti et al. 2015). In a
randomized, double-blind, placebo-controlled trial, curcumin
treatment (500 mg twice daily) for 4 to 8 week provides partial
improvement in people with major depressive disorder (Lopresti
et al. 2014). A recent meta-analysis data suggest that, curcumin
supplementation appears to be efficacious, safe and
well-tolerated anti-depressant and anxiolytic in patients (Ng et
al. 2017). In animal study, curcumin treatment is reported to
attenuate depressive phenotype during chronically stressed
condition via several mechanisms viz., reduction in adrenal
gland to body weight ratio, reduction in serum corticosterone
level, reduction in adrenal cortex thickness as well as
upregulation of BDNF and COX-2 expression and reduction in
(pCREB/ CREB) levels in brain. Curcumin administration increased
the level of synaptophysin and BDNF in amygdala alongside
reduced depressive like behavior in chronically stressed rats
(Zhang et al. 2014). Curcumin treatment is known to inhibit the
release of glutamate in synaptosome and induce activation of
GluN2B N-methyl-D-aspartate receptor (NMDAR) subunits resulting
in antidepressant like action (Zhang et al. 2013c; Lin et al.). Curcumin administration significantly reduced anxiety
like effect in ovariectomized (Morrone et al. 2016) and stressed
rats (Haider et al. 2015). The general mechanism of action of
curcumin treatment includes, inhibition of brain monoamine
oxidase (MAO)-A/ B activity, modulation of serotonin receptor,
amelioration of brain dopamine, serotonin and noradrenaline
levels, increase the neurotrophic factor, enhance neuronal
growth, increase neuroprotection, reduce neuroinflammation,
apoptosis and oxidative stress (Lopresti 2017; Choi et al.
2017).
|
| How may
Curcumin work as an antioxidant? |
|
The chemical structure of curcumin
gives it a powerful antioxidant capacity, which is 2.75 times
that of vitamin C and 1.6 times that of vitamin E.
Curcumin can help the body rid itself of hydroxyl radicals,
singlet oxygen, superoxide radicals, nitrogen dioxide, and NO.
Curcumin pretreatment was proven to reduce ischemia-induced
mutations in the heart (Dikshit et al.). The efficiency of
curcumin on endothelial heme oxygenase-1 (inducible stress
protein) employing bovine aortic endothelial cells was
discovered in an in vitro investigation that resulted in
increased cellular resistance to oxidative stress. Curcumin can
also help Caenorhabditis elegans live longer by lowering
intracellular ROS and lipofuscin levels during aging (Liao et
al., 2011). Previous research into the potential of C. longa to
sustain hippocampal cells of male Wistar rats from lead-induced
damage and reduces lipid peroxidation caused by toxic heavy
metals. Resveratrol and curcumin alleviate and synergistically
repair oxidative stress to the tissues by enhancing antioxidant
response through free radical scavenging (Al-Basher et al.,
2020). In one of the earlier studies, the anti-inflammatory and
antioxidant capability of curcumin was detected to be
synergistically enhanced with quercetin, and a synergistic
protective effect was also demonstrated in diazinon-induced rats
(Abdel-Diam et al., 2019). The anti-inflammatory impact of
berberine and curcumin may decrease oxidative stress, liver
inflammation, and lipid metabolism (Feng et al., 2018), and the
berberine combination also reduced inflammatory and oxidative
stress responses in the cortex and hippocampus of rats (Lin et
al., 2020). Antioxidant
and anti-inflammatory properties are the two primary mechanisms
that explain the majority of the effects of curcumin on the
various conditions. The anti-oxidative action of curcumin is
mediated through inhibition of stress-induced elevated levels of
8-hydroxydeoxyguanosine and 8-nitroguanine, regulating the
activity of mitochondrial respiratory complexes and upregulation
of Nrf2 (nuclear factor erythroid-derived 2-related factor 2)
that induces haemoxygenase-1 (HO-1) The anti-oxidant activity of
curcumin is predominantly due to the hydroxyl group. Curcumin
(5,10,20 and 30 µM) stimulates the expression of Nrf2 in a
concentration- and time-dependent manner, which in turn
increases HO-1 expression and HO-1 activity, which is a
redox-sensitive inducible protein that protects from various
forms of stress in cultured renal epithelial cells from rats. It
stimulates ARE (antioxidant responsive elements) binding
activity in NRK cells from rat kidney. Sreejayan et al. showed
that curcumin at a dose of 25 µM reduced nitrite production from
incubated solution of sodium nitroprusside in phosphate-buffered
saline. The scavenging of nitric oxide (NO) by curcumin was
concentration-dependent (50% at 20.4 and 100% at 50 µM).
Curcumin was shown not to interact with nitrite detection assay
or directly interact with nitrite. All forms of curcumin—demethoxy
curcumin, bisdemethoxy curcumin and diacetyl curcumin—had NO
scavenging property irrespective of the methoxy or the phenolic
group.Curcumin has also been shown to improve systemic
markers of oxidative stress. There is evidence that it can
increase serum activities of antioxidants such as superoxide
dismutase (SOD). A recent systematic review and meta-analysis of
randomized control data related to the efficacy of
supplementation with purified curcuminoids on oxidative stress
parameters—indicated a significant effect of curcuminoids
supplementation on all investigated parameters of oxidative
stress including plasma activities of SOD and catalase, as well
as serum concentrations of glutathione peroxidase (GSH) and
lipid peroxides. It is noteworthy to point out that all of the
studies included in the meta-analysis utilized some sort of
formulation to overcome bioavailability challenges, and four out
of the six used piperine. Curcumin’s effect on free radicals is
carried out by several different mechanisms. It can scavenge
different forms of free radicals, such as reactive oxygen and
nitrogen species (ROS and RNS, respectively); it can modulate
the activity of GSH, catalase, and SOD enzymes active in the
neutralization of free radicals; also, it can inhibit
ROS-generating enzymes such as lipoxygenase/cyclooxygenase and
xanthine hydrogenase/oxidase. In addition, curcumin is a
lipophilic compound, which makes it an efficient scavenger of
peroxyl radicals, therefore, like vitamin E, curcumin is also
considered as a chain-breaking antioxidant. Curcumin can
be served as a free radical scavenger in the body and also
promotes the endogenous antioxidant glutathione (GSH) synthesis
to protect cells or tissues from free radical injury. In vitro
cell and animal experiments also show that curcumin can enhance
the activity of superoxidase dismutase (SOD) and increase GSH
levels in cells and serum as well. In preclinical studies and
clinical trial, when the body organs or tissues become ischemia
due to the temporary interruption of blood circulation, such as
stroke, myocardial infarction, surgery, or transplantation,
etc.. After restoring blood flow, those ischemic reperfusion
tissues often produce excessive free radicals and cause
oxidative stress and injury. Administration of curcumin can
scavenge free radicals; thereby, reducing the damage of free
radicals to tissue cells, which also reduces damage caused by
excessive inflammation of tissues. Remarkably, investigating the role of
natural substances such as curcumin or derivatives with high
antioxidant potential that counteract oxidative stress seems to
be an effective preventive measure against free radical-linked
aging. Due to its chemical structure, curcumin has proved to be
an excellent scavenger of ROS and reactive nitrogen species and
is able to attenuate or prevent the exercise-induced oxidative
stress and inflammation, by modulation of GSH, catalase, and SOD
enzymes and inhibiting of ROS-generating enzymes such as
lipoxygenase / cyclooxygenase and xanthine hydrogenase/oxidase.
This has strengthened our conviction that curcumin is the golden
nutraceutical with proven potential in preventing/delaying the
onset of age-related diseases. Curcumin displays potent
biological and pharmacological effects on renal health. Aging is
an independent risk factor increasing the likelihood of
developing cardiovascular diseases which is due primarily to the
arteries remodeling and the development of vascular endothelial
dysfunction. Another promising anti-aging potential of curcumin
supplementation was shown in healthy middle-aged older men and
postmenopausal women. Indeed, 12 weeks curcumin administration
has improved resistance artery endothelial function by
increasing NO bioavailability and reducing vascular oxidative
stress. This suggests the critical role of curcumin to maintain
health vascular endothelium with aging, a fundamental element in
the prevention of atherosclerosis and arterial diseases. Another
study provides additional support about the role of curcumin
associated with aging in patients at risk of cardiovascular
diseases through reducing serum LDL-cholesterol and triglyceride
levels. Determining the long-term benefits of curcumin in patients
with cardiovascular diseases or at risk to develop
cardiovascular disorders seems like a promising research
avenue. The accelerated aging induced by oxidative stress
results in sex-specific differences in longevity and
susceptibility to age-related neurodegeneration. In a
previous research, curcumin was shown to prolong lifespan of
fruit fly model (Drosophila
melanogaster) through enhancing SOD activity.
These findings were corroborated by other data where
curcumin induced sex-specific in vivo responses to oxidative
stress. This includes protection from hydrogen peroxide and
alterations in behavior of
Drosophila melanogaster. This may rely on gene
expression and support the anti-aging role of curcumin in
gender-dependent manner. Curcumin belongs to the class of
hormetic agents that stabilize Nrf2 and enhance expression of
HO-1. Curcumin triggers Nrf2 pathway, which has a pivotal role
in activating antioxidant enzymes, such as thioredoxin
reductase, Hsp70, sirtuins. Furthermore, another study finding
reported that curcumin increased the activity of several
antioxidant enzymes including protein thiol, non-protein thiol,
GPx, and SOD in dogs fed with curcumin on day 30 compared with
control dogs. In addition, curcumin consumption stimulated the
antioxidant capacity in the serum of dogs and consequently
reducing ROS levels. Curcumin improved animal health, with
particular emphasis on the stimulation of the antioxidant system
and evidence of an anti-inflammatory effect. This suggested that
curcumin exerts beneficial effect on both growth, health and
consequently slowing down aging.
Curcumin supplementation accompanied with regular physical
exercise could potentially slowing down aging and/or preventing
oxidative stress-induced age-related functional and structural
changes and the age-related disorders. Collectively, these
findings reinforce the antioxidant potential of curcumin on
organ health function in the context of aging. Further
investigations are warranted to unravel the exact molecular
targets and signaling pathways responsible for the antioxidant
effects of curcumin in different human populations. |
| How may Curcumin work
against retinal diseases such as uveitis, Diabetic retinopathy, and
Age-related macular degeneration? |
|
|
Effect of Curcumin on Uveitis |
Curcumin administration attenuated the degenerative and
inflammatory conditions associated with eye like uveitis.
Corticosteroids are normally used for treatment of uveitis.
However, the adverse effects associated with these drugs limit
their use. One study evaluated the efficacy of curcumin against
chronic anterior uveitis. Curcumin was administered orally to
patients with chronic anterior uveitis at a dose of 375 mg three
times a day for 12 weeks. All patients who received
curcumin alone exhibited improvement, the group receiving
anti-tubercular therapy along with curcumin had a response rate
of 86%. Furthermore, follow-up of all patients for the next 3
years found recurrence rates of 55% for the first group and 36%
for the second group. The efficacy of curcumin on
recurrences after treatment was comparable to that of
corticosteroid therapy. Furthermore, lack of any adverse effects
with curcumin was an advantage over corticosteroid therapy.
Thus, the study demonstrated the therapeutic role of curcumin
and its efficacy against recurrent anterior uveitis.
Effect of Curcumin on
Diabetic Retinopathy | Retina, because of its high
content of polyunsaturated fatty acids (PUFA), high oxygen and
glucose uptake is, vulnerable to oxidative stress. Inflammation
is another underlying factor in the pathogenesis of diabetic
retinopathy . Oxidative stress leads to formation of ROS, which
is hypothesized to cause the development of neuropathy,
nephropathy, myocardial infarction and retinopathy.
Autooxidation of glucose, shift in redox balance, decrease in
the concentration of reduced glutathione (GSH–ROS scavenger),
vitamin C, Beta carotene and vitamin E and impairment of
antioxidant enzymes like superoxide dismutase (SOD), glutathione
reductase, glutathione peroxidase and catalase are considered as
the possible sources of oxidative stress in diabetes. Retina of
diabetic rats shows elevated superoxide and hydrogen peroxide
(H2O2) levels along with lipid peroxidation and oxidative damage
to DNA because of ROS. The other pathways that lead to diabetic
retinopathy are polyol pathway that depletes nicotinamide
adenine dinucleotide phosphate (NADPH) essential for
regeneration of GSH, advanced glycosylation end product (AGE)
and its receptor RAGE that get deposited in the retinal
capillary cells leading to more ROS and activation of NFKB and
caspase-3-induced apoptosis and damage to cellular constituents,
protein kinase C (PKC) pathway, which gets activated by
increased ROS and diacylglycerol as a result of hyperglycaemia
that increase vessel permeability and blood flow, stimulate
neovascularization, endothelial proliferation and apoptosis by
regulating the action of vascular endothelial growth factor
(VEGF), insulin-like growth factor-1 (IGF-1) and transforming
growth factor-β (TGF-β). ROS also activates hexosamine pathway
by inhibiting glyceraldehyde-3-phosphate dehydrogenase (GAPDH),
which further activates AGE pathway and the damage thereafter.
ROS causes dysfunction of mitochondria, which leads to
superoxide production and free radical damage and mutations in
mitochondrial DNA that leads to mitochondrial DNA damage in
retina in diabetes. Also, ROS-induced damage to mitochondria
suppresses antioxidant-mediated effective scavenging of ROS.
Exposure of pericytes and endothelial cells shows an increase in
caspase-3 activity, oxidative stress and transcription factors
that leads to capillary cell death. Photoreceptors, Muller
cells, ganglion cells and astrocytes are affected and are
involved in the pathogenesis of diabetic retinopathy.
Effect of Curcumin on
Age-related Macular Degeneration | Curcumin protected
against H2O2-induced cell death in a concentration of 10 µM when
pre-treatment time was less than 8–12 h. Curcumin reduced and
maintained intracellular ROS levels in age-related macular
degeneration-RPE cells at varied concentrations (0.1, 1, 10 µM)
for 12 h in H2O2-exposed cells. Treatment with curcumin showed
increase of anti-oxidant genes HO-1, SOD2 (superoxide dismutase
2) and GPX1 (glutathione peroxidase 1) and reduces the
expression of VEGF, PDGF (platelet-derived growth factor) and
IGFBP 2 (insulin-like growth factor binding protein 2) using
RTPCR in RPE-age-related macular degeneration cells.
Pre-treatment with curcumin inhibited JNK pathway that involves
a series of inflammatory pathways leading to cell death in
RPE-age-related macular degenerationcells. Thus, curcumin may
potentially be an ideal drug in restoring the function in
age-related macular degeneration patient-derived RPE cells. Park
et al. studied the protective effects of curcumin in
A2E-accumulated ARPE-19 cells that were exposed to blue light to
induce cytotoxicity. A2E and iso-A2E are main pigments of
lipofuscin that accumulate in RPE and cause RPE cell death in
age-related macular degeneration. |
| How may Curcumin work
as an Anti-inflammatory? |
|
|
Chronic inflammation may be the engine that drives many of the
most feared illnesses of middle and old age. This concept
suggests a new and possibly much simpler way of warding off
disease. Instead of different treatments for, say, heart
disease, Alzheimer's and colon cancer, there might be a single,
inflammation-reducing remedy that would prevent all three.
Curcumin activity for inflammation after giving oral
administration was comparable to that of cortisone or
phenylbutazone. Curcumin reduced inflammatory swelling, this
effect resulted from inhibiting biosynthesis of inflammatory
prostaglandins from arachidonic acid and neutrophil function
during inflammatory states. A large number of studies have proved that curcumin
has a variety of biological activities, among which
anti-inflammatory effect is a significant feature of it. The physiological and pathological
mechanisms of inflammatory bowel disease, psoriasis,
atherosclerosis, COVID-19 and other research focus diseases are
not clear yet, and they are considered to be related to
inflammation. The anti-inflammatory effect of curcumin can
effectively improve the symptoms of these diseases. The
anti-inflammatory property of curcumin is by inhibition of
TNF-dependent NFkB (tumour necrosis factor-dependent
transcriptional nuclear factor kappa B) and pathways that
produce reactive oxygen intermediates. Curcumin downregulates
COX-2 (cyclooxygenase-2) that are predominantly seen at the
sites of inflammation that mediate pain and inflammatory
process. Curcumin is effective against inflammation and edema.
Curcumin is acceptable at large doses (12 g/day) in humans,
according to phase I clinical studies and has been shown to have
medicinal potential against a variety of human ailments,
particularly diabetes, cancer, arthritis, cardiovascular
disease, Crohn’s disease, and neurological disease.
Curcumin was evaluated for its anti-inflammatory potential in a
rat model for the treatment of osteoarthritis. Results suggested
that curcumin significantly reduced the expression of cytokine
levels in synovial fluid targeting the TLR4/NF-κB signaling
pathway. The effect of curcumin on inflammatory indices was
evaluated in a randomized control study. Results of the study
indicated an outstanding reduction in inflammation through
reduction in TNF-α, concluding that curcumin plays a key role in
inflammation suppression in hepatic patients with nonalcoholic
fatty liver disorders. The findings of another study revealed
that curcumin reduced the expression of IL-6, TNF-α, and the
NF-κB signaling pathway and reduced the rate of cell apoptosis
resulting in the healing of injured kidney cells. Apart from
individual therapy, curcumin in combination therapy has shown
significant anti-inflammatory action. In this regard,
hyperlipidemia-induced inflammation was targeted by the combined
delivery of curcumin and rutin in Wistar rats. Results of the
study showed an increase in HDL and a decrease in triglyceride
level after treatment with curcumin and rutin combined therapy.
It is concluded that curcumin has potential in treating
inflammation and can be used as a therapeutic medicament. Curcumin inhibits pro-inflammatory enzyme 5-LOX
(5-lipoxygenase) that are involved in the biosynthesis of
leukotrienes and lipid mediators of inflammation. It also
downregulates inflammatory cytokines like TNF, IL-1
(interleukin-1), IL-6, IL-8, iNOS (inducible nitric oxide
synthase) and interferon-ϒ. Curcumin at a dose of 360 mg/dose
for 3–4 months in humans reduced clinical relapse in those with
quiescent inflammatory bowel disease and decreased the use of
concomitant medications. This
inflammation theory explains how immune-system errors are
linked to more illnesses. Medical researchers are becoming
increasingly convinced that the most primitive part of the
immune system (inflammation), may play a crucial role in some of
the most devastating afflictions of modern humans, including
heart disease, cancer, diabetes and possibly Alzheimer's.
Study findings suggest that in the past, gene
variants rose in frequency in the human population to help
protect us against viruses, bacteria and other pathogens. But
now in our modern world, the environment and exposure to
pathogens has changed, and the genetic variants that were
originally meant to protect us, now make an autoimmune reaction
more likely. These results are consistent with the hygiene
hypothesis in which our cleaner environment is thought to
contribute to the increasing prevalence of inflammatory
diseases.
While short-term inflammation in the body is a necessary component of a
functioning system, helping to fight off pathogenic invasion and repairing
tissue and muscle damage, chronic inflammation is widely attributed with
almost every disease known to the Western world. This includes heart
disease, cancer and a whole host of neurological disorders. Curcumin reduces
inflammation by lowering histamine levels and by
increasing the production of natural cortisone by the adrenal
glands. Extensive research over the past 30 years has shown that
curcumin plays an important role in the prevention and treatment
of various pro-inflammatory chronic diseases including
neurodegenerative, cardiovascular, pulmonary, metabolic,
autoimmune and malignant diseases. Oral administration of curcumin in instances of acute
inflammation was found to be as effective as cortisone or
phenylbutazone, and half as effective in cases of chronic
inflammation. Its anti-inflammatory properties may be attributed
to its ability to inhibit both biosyntheis of inflammatory
prostaglandins from arachidonic acid and neutrophil function
during inflammatory states. Oxidative stress has been implicated
in many chronic diseases, and its pathological processes are
closely related to those of inflammation, in that one can be
easily induced by another. In fact, it is known that
inflammatory cells liberate a number of reactive species at the
site of inflammation leading to oxidative stress, which
demonstrates the relationship between oxidative stress and
inflammation. In addition, a number of reactive oxygen/nitrogen
species can initiate an intracellular signaling cascade that
enhances pro-inflammatory gene expression. Inflammation has been
identified in the development of many chronic diseases and
conditions. These diseases include Alzheimer’s disease (AD),
Parkinson’s disease, multiple sclerosis, epilepsy, cerebral
injury, cardiovascular disease, metabolic syndrome, cancer,
allergy, asthma, bronchitis, colitis, arthritis, renal ischemia,
psoriasis, diabetes, obesity, depression, fatigue, and acquired
immune deficiency syndrome (AIDS). Clinical trials have also
shown that curcumin can reduce inflammatory mediators. The
regulatory effect of curcumin on immune cells is beneficial to
its treatment of inflammatory diseases. Curcumin mainly acts on
dendritic cells, T helper 17 cell, T regulatory cell. Curcumin
inhibits Th17 differentiation, and regulate Treg/Th17 rebalance
is by inhibit the IL‑23/Th17 pathway.29,30 Oxidative stress is
closely related to inflammatory processes. Curcumin reduces ROS
production due to its effect on nicotinamide adenine
dinucleotide phosphate (NADPH) oxidase and increasing the
activity of antioxidant enzymes, and is related to Nrf2-Keap1
pathway. Curcumin reduces inflammation through its antioxidant
activity. Curcumin has significant anti-inflammatory effects,
and a large number of preclinical or clinical researches have
studied its effect on inflammatory diseases, among them,
inflammatory bowel disease, arthritis, psoriasis, depression,
atherosclerosis and COVID-19 are the focus of research hotspots.
Current evidences suggest that curcumin is effective in reducing
levels of inflammatory mediators, and that curcumin’s
anti-inflammatory properties may have a beneficial effect on
these diseases. Tumor necrosis factor α
(TNF-α) is a major mediator of inflammation in most diseases,
and this effect is regulated by the activation of a
transcription factor, nuclear factor (NF)-κB. Whereas TNF-α is
said to be the most potent NF-κB activator, the expression of
TNF-α is also regulated by NF-κB. In addition to TNF-α, NF-κB is
also activated by most inflammatory cytokines; gram-negative
bacteria; various disease-causing viruses; environmental
pollutants; chemical, physical, mechanical, and psychological
stress; high glucose; fatty acids; ultraviolet radiation;
cigarette smoke; and other disease-causing factors. Therefore,
agents that downregulate NF-κB and NF-κB–regulated gene products
have potential efficacy against several of these diseases.
Curcumin has been shown to block NF-κB activation increased by
several different inflammatory stimuli. Curcumin has also been
shown to suppress inflammation through many different mechanisms
beyond the scope of this review, thereby supporting its
mechanism of action as a potential anti-inflammatory agent. The high
concentrations of curcumin contained in turmeric act to target multiple
steps in the inflammatory pathway, blocking inflammatory markers at the
molecular level and thereby significantly lowering long-term inflammation in
your body.
Whether we are healing from an injury or an autoimmune disease, inflammation
is a common health challenge. Nutritionists, functional medicine GP’s and
physiotherapists commonly use curcumin for it’s anti-inflammatory qualities.
And it’s no wonder considering
six major scientific trials all found curcumin to possess a potent
anti-inflammatory action, which is completely non-toxic in nature. Recent findings suggest dietary
interventions, including curcumin supplementation, as a strategy
to combat inflammaging. Interestingly, the age-modulatory
properties and healthful effects of curcumin have been
illustrated in different cellular and animal models, including
C. elegans, Drosophila, and mice. As it was clearly discussed
above, curcumin was found to extend both healthspan and
lifespan, mainly blocking the most relevant proinflammatory
pathway NF-kB. In addition to the well-documented
evidence supporting the numerous biological properties of
curcumin in inhibiting NF-κB signaling dependent inflammation.
Indeed, curcumin was shown
to modulate the senescence-associated secretory phenotype
(SASP), which characterizes senescent cells and contributes to
fuel the inflammaging. Interestingly, the short-term
treatment of cells with low concentrations of curcumin decreased
the level of secreted pro-inflammatory cytokines such as IL-8 in
normal young cells. Moreover, lower doses of curcumin have
increased the production of sirtuin, i.e., NAD-dependent
deacetylases, and sirtuin 1 reduced inflammation by inhibiting
NF-κB signaling. Curcumin inhibits and regulates tissue
production and secretions of pro-inflammatory cytokine, such as
interleukin-4 (IL-4)
tumor necrosis factor alpha (TNF-α). Conversely, curcumin
can increase anti-inflammatory
cytokine production, such as IL-10 and soluble
intercellular adhesion molecule 1 (sCAM-1). In
preclinical studies, curcumin can reduce the degree of
inflammation of animal skin and prevent or reduce the
respiratory tract inflammation caused by viral or bacterial
infections. In clinical trials, the curcumin treatment improves
pain symptoms caused by
osteoarthritis and tissue inflammation and delays the
degradation of
articular cartilage, which improves the mobility and quality
of life in patient. Furthermore, curcumin also recovers the
effect of nicotine,
acetylcholine, serotonin,
barium chloride, and histamine on the reduction of
intestinal peristalsis. It
is
believed that curcumin exerts its effect in dose-dependent and
cell-context manner on the protein activity involved SASP.
Particularly, increasing evidence suggests that repeated
stimulation of innate immune responses over time results in the
development of inflammaging. In these settings, both an
increased burden of senescent cells during aging and a
hyper-stimulation of macrophages over time can play key roles of
inflammaging process. Recent reports of randomized controlled
trials conducted from 2008–2020 have demonstrated that curcumin
was able not only to modulate the antioxidant status but also
restore quantity, quality, and functional-metabolic status of
immune cells. This lends support to other data showing partial
anti-inflammatory, immunotropic and antioxidant activity of
turmeric extract in vitro and in vivo. Further implication of
curcumin in modulating aging-related inflammation through
lowering CRP level in dose-dependent manner in rats’ model was
reported. Moreover, MDA and NO levels were increased
significantly in animals fed with curcumin. This has
strengthened our belief that curcumin slows down the aging
process by suppressing age-related inflammatory indices. A
study
evaluating several pharmaceutical anti-inflammatories found that aspirin and
ibuprofen are the least potent, while
curcumin is among the most potent anti-inflammatory and
anti-proliferative agents available. Inflammation is
thought to be one of the major factors in all kinds of diseases, and
turmeric contains loads of curcumin, a powerful anti-inflammatory substance. It's been shown
to alleviate joint pain, and can even help with
heartburn and
indigestion. Researchers are also looking at curcumin for its
anti-aging properties.
Turmeric is safe and non-toxic and has been studied for
anti-inflammatory properties, inhibiting various molecules that
contribute to inflammation such as lipooxygenase, COX-2,
leukotrienes, prostaglandins, nitric oxide, interferon-inducible
protein, tumor necrosis factor (TNF), and interleukin-12
(IL-12). One study compared the effectiveness of Curcumin – the
active ingredient in turmeric – and a popular non-steroidal
anti-inflammatory drug (NSAID) called phenylbutazone. At the end
of the six days, those taking the Curcumin and the NSAID enjoyed
a significantly better anti-inflammatory response than placebo.
Curcumin in inflamed organs (liver, lung, brain and kidneys)
reduces the expression levels of NLRP3, IL‐1β, IL‐18 and
caspase‐1 and inhibits the inflammasome. Curcumin activated Nrf2
and inhibited NF‐kB. In the liver, curcumin directly targets
3'UTR‐rTXNIP with the help of miR200a and inhibits NLRP3
inflammasome. Curcumin reduces the severity of neurotoxicity by
inhibiting the formation of TXNIP/NLRP3 complex associated with
ER stress through the regulation of AMPK. Curcumin in
LPS‐stimulated mouse macrophages inhibits the activity of NLRP3
inflammasome by inhibiting potassium excretion, mitochondrial
instability, ASC oligomerization and speckle formation. In
addition to the above, ROS, autophagy, Sirtuin‐2 and acetylated
alpha‐tubulin are the targets used by curcumin in inhibiting the
inflammasome. In the lungs, curcumin effectively prevented the
increasing Notch1. In addition to inflammasome components,
curcumin effectively inhibits TLR4 and MyD88 expression and IBB
phosphorylation. Curcumin has a regulatory effect on several
molecules in the intracellular signal transduction pathways
involved in inflammation. |
|
According to Sandur et al. (2007), curcumin, demethoxycurcumin,
and bisdemethoxycurcumin are the active compounds in
C.
longa that inhibit TNF-induced NF-κB activation.
Researchers discovered that curcumin has anti-inflammatory
properties by inhibiting the pro-inflammatory transcription
factor (NF-κB). Curcumin also inhibits the binding of activator
protein 1 (AP-1) binding factors, but the Sp1 binding factor
remained unaffected. Curcumin inhibits the activation of NF-κB
by phorbol ester and hydrogen peroxide, in addition to TNF-α.
Furthermore, curcumin suppresses the NF-κB activation pathway
after the convergence of multiple stimuli but before human I
kappa B alpha phosphorylation. Curcumin is equally
efficacious as cortisone or phenylbutazone when given orally in
acute inflammation. Curcumin’s therapeutic effect in sepsis
appears to be achieved by activation of peroxisome
proliferator-activated receptor gamma (PPAR-γ), which leads to
inhibition of pro-inflammatory cytokine along with expression
and release of TNF-α (Jacob et al., 2008). Majority of the
benefits seemed to be due to the anti-inflammatory and
antioxidant properties of curcumin, while the quercetin in the
molecule was negligible. Interestingly, anti-inflammatory effects of curcumin have been
shown to encompass the inhibition of MCP-1. Other
anti-inflammatory effects involved the downregulation of
inflammatory mediators such as COX-2 activity, lipoxygenase,
iNOS, MAPK, JAK and inhibition of TNF-α production, IL-1, -2,
-6, -8, and -12, macrophage migration inhibitory factor (MIF). A
recent study has shown that curcumin not only stimulated the
antioxidant system and reduced oxidative reactions in dogs but
also reduced leukocyte counts, which suggests mild
anti-inflammatory effects achieved in dogs fed with at a dose of
30 mg of curcumin/dog/day, These substantiate previous findings,
where it was observed that nursing lambs fed with curcumin had
lower total leukocytes, neutrophils, and lymphocytes. A similar
effect was reported in rats treated with 50 and 400 mg/kg
curcumin, indicating a remarkable improving effect on health and
the immune response. This points toward the importance of
curcumin in reversing the inflammatory responses and enhancing
the immune system performance, both playing a critical role in
ameliorating health and consequently slowing down aging (see
Figure 2).Curcumin has also been shown to
inhibit mediators of the inflammatory response, including cytokines,
chemokines, adhesion molecules, growth factors, and enzymes like
cyclooxygenase (COX), lipoxygenase (LOX), and inducible nitric oxide
synthase (iNOS). Nuclear factor-kappa B (NF-κB) is a transcription factor
that binds DNA and induces the transcription of the COX-2 gene, other
pro-inflammatory genes, and genes involved in cell proliferation, adhesion,
survival, and differentiation. The anti-inflammatory effects of curcumin
result from its ability to inhibit the NF-κB pathway, as well as other
pro-inflammatory pathways like the mitogen-activated protein kinase (MAPK)-
and the Janus kinase (JAK)/Signal transducer and activator of transcription
(STAT)-dependent signaling pathways. Inhibition of dextran sulfate sodium
(DSS)-induced colitis by curcumin in mice has been associated with a
downregulation of the expression of p38-MAPK and pro-inflammatory cytokine
TNF-α and a reduction of myeloperoxidase (MPO) activity, a marker of
neutrophil infiltration in intestinal mucosa. Curcumin has also been shown
to improve colitis by preventing STAT3 activation and STAT3-dependent
induction of cell proliferation in mouse colon. Moreover, curcumin was shown
to attenuate the immune response triggered by collagen injections in a mouse
model of rheumatoid arthritis, partly by blocking the proliferation of T
lymphocytes in mouse splenocytes. In addition, curcumin has been found to
reduce the secretion of TNF-α and IL-1β and the production of COX-2-induced
prostaglandin G2. In one study, curcumin inhibited the secretion of matrix
metalloproteins (MMPs) — responsible for the degradation of the synovial
joints — in human fibroblast-like synoviocytes and in human articular
chondrocytes. Curcumin has also been found to alleviate neuro-inflammation
in a mouse model of traumatic brain injury, reducing macrophage and
microglial activation and increasing neuronal survival. A placebo-controlled
trial in 40 men who had surgery to repair an inguinal hernia or hydrocele
found that oral curcumin supplementation (1.2 g/day) for five days was more
effective than placebo in reducing post-surgical edema, tenderness and pain,
and was comparable to phenylbutazone therapy (300 mg/day). Scientists now
believe that chronic, low-level inflammation plays a major role
in almost every chronic, Western disease. This includes heart
disease, cancer, metabolic syndrome, Alzheimer's and various
degenerative conditions. Therefore, anything that can help fight
chronic inflammation is of potential importance in preventing
and even treating these diseases. Curcumin is strongly
anti-inflammatory. In fact, it’s so powerful that it matches the
effectiveness of some anti-inflammatory drugs, without the side
effects. It blocks NF-kB, a molecule that travels into the
nuclei of your cells and turns on genes related to inflammation.
NF-kB is believed to play a major role in many chronic diseases.
The key takeaway is that curcumin is a bioactive substance that
fights inflammation at the molecular level. A 1999 study
published in the journal Phytotherapy Research found that the
primary polyphenol in turmeric, the saffron colored pigment
known as curcumin, compared favorably to steroids in the
management of chronic anterior uveitis, an inflammatory eye
disease. A 2008 study published in Critical Care Medicine found
that curcumin compared favorably to the corticosteroid drug
dexamethasone in the animal model as an alternative therapy for
protecting lung transplantation-associated injury by
down-regulating inflammatory genes. An earlier 2003 study
published in Cancer Letters found the same drug also compared
favorably to dexamethasone in a lung ischaemia-repurfusion
injury model. A 2004 study published in the journal Oncogene
found that curcumin (as well as resveratrol) were effective
alternatives to the drugs aspirin, ibuprofen, sulindac,
phenylbutazone, naproxen, indomethacin, diclofenac,
dexamethasone, celecoxib, and tamoxifen in exerting
anti-inflammatory and anti-proliferative activity against tumor
cells. Curcumin down‐regulates the expression of inflammatory
enzymes, such as COX2 and iNOS, inhibits the expression of the
5‐LOX pro‐inflammatory enzyme and chemokines and reduces the
expression of CRP and inflammatory cytokines of TNF‐α, IL‐6 and
IL‐8. Oral curcumin supplementation may potentially play a role in
inhibiting the COVID‐19 inflammation along with other drug
regimens by affecting these pathways and molecules and due to
applying anti‐inflammatory, antioxidant and anti‐apoptotic
properties without specific side effects. The spice worked as well as the
drug, but without the negative side effects.
Because of the crucial role of inflammation in most chronic diseases, the
potential of Curcumin
has been examined in neoplastic, neurological, cardiovascular, pulmonary and
metabolic diseases. The pharmacodynamics and pharmacokinetics of
Curcumin
have been examined in animals and in humans.
Clinically, chronic curcumin administration (375 mg, t.i.d., p.o., for
6–22 months) reduced the symptoms associated with idiopathic
inflammatory orbital pseudo-tumors in patients (Lal et al.
2000). In a study of curcumin’s anti-inflammatory
properties, Satoskar et al. evaluated the effects of this
polyphenol on spermatic cord edema and tenderness in 46 men
(15–68 years old) who had just undergone surgical repair of an
inguinal hernia and/or hydrocele. After surgery, patients were
randomly assigned to receive curcumin (400 mg), placebo (250 mg
lactose powder), or phenylbutazone (100 mg) three times a day
for 6 days. Curcumin proved to be superior by reducing all four measures of
inflammation. Curcumin binds to Toll-like receptors (TLRs) and
regulates downstream nuclear factor kappa-B (NF-κB),
Mitogen-activated protein kinases (MAPK), Activator Protein
1(AP-1) and other signaling pathways. Curcumin can down-regulate NF-κB through acting on Peroxisome
proliferator-activated receptor gamma (PPARγ). Curcumin can also
play anti-inflammatory effects by regulating The Janus
kinase/Signal transducer and activator of transcription
(JAK/STAT) inflammatory signaling pathway. Curcumin could
directly restrain the assembly of NLRP3 inflammasome, or
inhibits the activation of NLRP3 inflammasome by inhibition of
NF-κB pathway, which may be one of the mechanisms of curcumin
for the treatment of inflammatory diseases. In the studies
of inflammatory cells and animals, curcumin decreased levels of
pro-inflammatory mediators such as Interleukin-1, Tumor necrosis factor-α (TNF-α),
Inducible nitric oxide synthase (iNOS), NO, Regulated upon
activation normal T cell expressed and secreted factor(RANTES),
Granulocyte colony-stimulating factor (G‐CSF), and Monocyte
chemotactic protein‐1 (MCP-1). |
|
How may Curcumin work against CARDIOVASCULAR HEART Disease like
coronary atherosclerosis, hypertension, stroke, elevated ldl
cholesterol and triglyceride levels? |
Effect of Curcumin on Cholesterol and Triglyceride Levels
| Curcumin's
protective effects on the cardiovascular system include lowering
cholesterol and triglyceride levels, decreasing susceptibility
of low density lipoprotein (LDL) to lipid peroxidation, and
inhibiting platelet aggregation. In clinical researches,
curcumin is demonstrated to have the antihypertensive effects
while lowering blood pressure it can also increase myocardial
trophic blood flow. Curcumin increases VLDL cholesterol
trans-protein plasma, causing increased levels and
mobilization of α-tocopherol from adipose tissue that
protects against oxidative stress that occurs during
atherosclerosis. It was suggested that oral intake of 500 mg/day
curcumin for a week leads to a significant reduction in serum
lipid peroxide (33%) and total serum cholesterol (12%) levels
while increasing HDL cholesterol (29%). One study evaluated the effects of curcumin
in reducing the serum levels of cholesterol and lipid peroxides
in ten healthy human volunteers. Curcumin (at 0.5 g/day)
administered to the volunteers for 7 days reduced serum lipid
peroxides by 33% and total serum cholesterol levels by 11.63%,
and increased HDL cholesterol by 29%. Because of these
properties, curcumin was suggested to act as a chemopreventive
agent against atherosclerosis. Curcumin can reduce the viscosity of blood
and thrombosis formation via hindering the synthesis of
thromboxane A2 (TXA2) and regulating calcium signals to prevent
platelet activation and aggregation. Curcumin may affect
bleeding during menstruation and in repair of the endometrium,
because it can inhibit platelet aggregation. Therefore, it is
not proper to use during menstruation as it may cause excessive
menstrual blood volume and prolonged menstruation. In addition,
curcumin can inhibit the activation of NF-κB, AKT, and ERK to
protect and activate vascular endothelial cell from
incapacitation, which reduces arterial sclerosis, thrombosis,
and abnormal blood pressure. Clinical studies have shown that
curcumin reduces the recurrence rate in patients with coronary
artery obstruction disease and who have had installed vascular
stents within coronary artery. Turmeric extract demonstrated decreased susceptibility of LDL to
lipid peroxidation in addition to lower plasma cholesterol and
triglyceride levels. Higher doses decreased lipid peroxidation
of cholesterol and triglyceride levels. Curcumin's effect on
cholesterol levels may be due to decreased cholesterol uptake in
the intestines and increase conversion of cholesterol to bile
acids in the liver. Curcumin
may help reverse many steps in the heart disease process.
Perhaps the main benefit of curcumin when it comes to heart
disease is improving the function of the endothelium, which is
the lining of your blood vessels. It’s well known that
endothelial dysfunction is a major driver of heart disease and
involves an inability of your endothelium to regulate blood
pressure, blood clotting and various other factors. Several
studies suggest that curcumin leads to improvements in
endothelial function. One study found that it’s as effective as
exercise while another shows that it works as well as the drug
Atorvastatin. In addition, curcumin reduces
inflammation and oxidation (as discussed above), which play a
role in heart disease as well. One study randomly assigned 121
people, who were undergoing coronary artery bypass surgery,
either a placebo or 4 grams of curcumin per day, a few days
before and after the surgery. The curcumin group had a 65%
decreased risk of experiencing a heart attack in the hospital. A
study in
Nutrition Research in 2012, postmenopausal
women who took curcumin for eight weeks had an improvement in arterial
function, comparable to that seen in women who engaged in aerobic exercise.
Another
study in
Phytotherapy Research in 2013 found that
curcumin reduced triglycerides, while a
study in 2014 found that curcumin significantly reduced LDL
(“bad”) cholesterol and triglycerides in people with metabolic syndrome. Curcumin
also helps the endothelium (the lining of blood vessels) to function
at its optimum level, similar to the effect found during intense exercise.
Effect of Curcumin on Hypertension | Hypertension is a
condition in which the pressure on blood vessels is greater than
the normal pressure. A clinical study demonstrated that turmeric
(standardized to 22.1 mg of active curcumin) supplementation (3
capsules daily for three months) attenuated hematuria,
proteinuria and systolic blood pressure associated with
refractory or relapsing nephritis in patients without any
adverse events (Khajehdehi et al. 2012). In animal study,
curcumin administration downregulated the expression angiotensin
I receptor in vascular smooth muscle cells. In addition,
curcumin reduced angiotensin II-induced high blood pressure in
C57Bl/6J mice associated with downregulated expression of
angiotensin I receptor and decreased vasoconstriction in the
mesenteric artery (Yao et al. 2016). Further, curcumin
administration upregulated eNOS expression, decreased superoxide
enzyme level and downregulated p47phox NADPH oxidase expression
in vascular tissues, which is known to be responsible for
2kidney-1clip induced hypertension in rats (Boonla et al. 2014).
In another study, curcumin treatment increased the expression of
eNOS, decreased oxidative stress, restored glutathione redox
ratio in aortic tissues along with decrease in plasma protein
carbonyls, MDA and urinary nitrate/ nitrite levels in cadmium
intoxicated mice resulting in anti- hypertensive effect
(Kukongviriyapan et al. 2014). In conclusion, curcumin
supplementation effectively reduce hypertension via blocking
angiotensin I receptor, reducing circulating
angiotensin-converting enzyme, inducing vasodilation and
mediating nephroprotection. Stroke, sometimes called a “brain
attack”, occurs when blood circulation to a part of the brain is
blocked or ruptured. In animal studies, curcumin pre- and
post-treatment significantly improved CAT, glutathione
peroxidase (GPx) and SOD, while reduced TNF-a, IL-6, MDA and
xanthine dehydrogenase levels in forebrain tissue. In addition,
curcumin treatment significantly reduced apoptotic index induced
by bilateral common carotid artery occlusion/reperfusion in rats
(Altinay et al. 2017), increased the numbers of BrdU-positive
cells, BrdU/doublecortin-positive cells, activated notch
signaling pathway and stimulated neurogenesis during stroke (Liu
et al. 2016). Curcumin pretreatment (200 mg/kg, i.p., for 7
days) significantly decreased MDA, NO, TNF-a, IL-1b, caspase-3,
while increased SOD and GPx levels in the spinal cord of
ischemia-reperfusion injury in rats. Further, curcumin
administration reduced oxidative stress, inflammation and
apoptosis in spinal cord as well as reversed locomotor deficit
in rats (Gokce et al. 2016). Curcumin administration upregulated
eukaryotic initiation factor 4 A, adenosylhomocysteinase,
isocitrate dehydrogenase, ubiquitin carboxyterminal hydrolase
L1, while downregulated pyridoxal phosphate phosphatase
expressions in the cerebral cortex of rat (Shah et al. 2016a).
Curcumin treatment (50 mg/kg, i.p., for five days) downregulated
TNF-a, IL-6, Ac-p53 and Bax, while upregulated Bcl-2 and SIRT1
expression in brain. In addition, curcumin increased
mitochondrial cytochrome clevels, mitochondrial complex I
activity, mitochondrial membrane potential, while decreased
cytosolic cytochrome clevels in brain resulting in reversal of
mitochondrial dysfunction in transient middle cerebral artery
occlusion/reperfusion stroke model of rat (Miao et al. 2016).
Effect of Curcumin on Atherosclerosis |
Curcumin has an anti-atherosclerosis effect, possibly through
its anti-inflammatory properties.
Anti-hypercholesterolemic, anti-atherosclerotic (Gao et al.,
2019), and protective capabilities against cardiac ischemia and
reperfusion (Wang et al., 2018) of curcumin have been proven in
preclinical and clinical trials. Curcumin has anti-CVD potential
by improving the lipid profile of patients, and it might be
administered alone or as a dietary supplement to traditional CV
medicines (Qin et al., 2017). Curcumin is also seen in many
studies to protect against coronary heart disease (Li H. et al.,
2020) and also possesses anticoagulant properties. Curcumin limits the risk of
lipid peroxidation, which triggers inflammatory responses that
may lead to cardiovascular disease (CVD) and atherosclerosis,
due to its ability to scavenge reactive oxygen forms. Moreover,
curcumin and statins influence the same mediators of plasma
lipid changes. Experimental studies on atherosclerosis concluded
that the positive effects of curcumin on atherosclerosis were
associated with the dose of curcumin. Additionally, curcumin has
the ability to prevent endothelial dysfunction and smooth muscle
cell proliferation and migration. These properties
of curcumin are responsible for skewing macrophage polarization
from M1 to M2, regulating TLR4/MAPK/NF-κB pathways in
macrophages (which induce M2 polarization) and secreting
interleukins (IL-4 and/or IL-13). Moreover, curcumin may
indirectly maintain cell homeostasis by regulating the
expression and activity of lipid transporter, which is
responsible for cholesterol uptake and efflux. Zhou et al.
suggested that curcumin could be used as a therapeutic
supplement in atherosclerosis due to its ability to modulate
macrophage polarization through the inhibition of the toll-like
receptor (TL4)-mediated signaling pathway. This indicates that
curcumin is related to anti-inflammatory and atheroprotective
effects. Zhang et al. investigated the potential suppression of
atherosclerosis development by curcumin in ApoE-knockout mice by
inhibiting TLR4 expression in an animal model. Mice were fed a
high-fat diet supplemented with curcumin for 16 weeks and
compared to a control group (without curcumin supplementation).
The results indicated that, in vitro, curcumin at least
partially inhibited TLR4 expression, inhibited NF-κB activation
in macrophages, and, indeed, influenced the inflammatory
reaction. The causal role of curcumin in inhibiting TLR4
expression was also demonstrated by Meng et al. [46], who
indicated that its mechanism may be related to the blocking of
NADPH-mediated intra-cellular ROS production. Comprehensively,
the treatment of atherosclerosis and other cardiovascular
diseases with curcumin was shown to be effective in many
studies. Curcumin reduces the
activation of M1 macrophages. Curcumin regulates the polarization
and plasticity of macrophages by affecting TLR4/MAPK/NF-κB
pathway, which is beneficial to reduce atherosclerosis. In
ApoE−/− mice fed a high-fat diet supplemented with 0.1% curcumin
significantly decreased TLR4 expression in atherosclerotic
plaques and reduced the development of atherosclerosis. In
addition, curcumin supplementation can inhibit the activation of
NF-κB in aorta and the levels of IL-1β and TNF-α in aorta and
serum.122 Activation of the NF-κB pathway leads to activation of
NLRP3 inflammasome. Inhibition of NLRP3 inflammasome improves
atherosclerotic lesions in ApoE−/− rats,123 and
anti-inflammatory therapy targeting IL-1β reduces the recurrence
rate of cardiovascular events. Curcumin can inhibit
NF-κB-mediated NLRP3 expression, thereby inhibiting vascular
smooth muscle cell migration, and alleviating hypertension,
vascular inflammation and vascular remodeling in spontaneously
hypertensive rats, which is beneficial to cardiovascular
diseases including atherosclerosis.125 In ApoE−/− mice,
atorvastatin calcium and curcumin synergistically inhibited
adhesion molecules and plasma lipid levels, reducing foam cell
formation and inflammatory cytokines secretion by blocking
monocyte migration to the intima. Curcumin has significant
efficacy in the treatment of atherosclerosis in animal models.
Clinical evidence in non-atherosclerotic populations suggests
that curcumin can reduce lipid levels and inflammatory
responses, as it did in a mouse model. A meta-analysis of 20
randomized controlled trials with 1427 participants suggested a
significant decrease in plasma concentrations of triglycerides
and an elevation in plasma high-density lipoprotein cholesterol
(HDL-C) levels. In another randomized controlled
trial, administration of curcumin for 6 months increased the
level of adiponectin in serum, decreased pulse wave velocity and
reduced the level of leptin, uric acid, triglyceride, total body
fat, visceral fat and insulin resistance alongside lowered the
atherogenic risks in type 2 diabetic population (Chuengsamarn et
al. 2014). In animal study, curcumin administration reported to
possess anti-atherosclerotic activity by downregulating the
expression of lipocalin-2 in apolipoprotein E knockout mice (Wan
et al. 2016). Curcumin supplementation downregulated monocyte
chemotactic protein-1, P-selectin, vascular cell adhesion
molecule-1, intracellular adhesion molecule-1 and MMP (1, 2 and
9) expressions, exerting anti-atherosclerotic activity. It
oxidized LDL and lowered lipid levels in the serum of
hypercholesterolemic rabbits (Um et al. 2014). Another
mechanistic study revealed that curcumin supplementation
suppresses the expression of CD36 and aP2 in macrophages of
atherosclerotic mice (Hasan et al. 2014). In murine macrophage
line RAW264.7, curcumin reduced ox-LDL- induced TNF-a, IL-1b,
IL-6 production and apoptosis along with upregulation of
ATP-binding cassette transporter (ABCA1) and CD36 expressions,
thereby inducing lipid disposal and removal. Studies have shown
that endothelial dysfunction is a common cause of heart disease,
occurring when the endothelium is no longer able to regulate
blood pressure, clotting and a number of other factors.
Therefore, by improving endothelial function, curcumin lowers
your risk of heart disease. In addition to helping out the
endothelium, curcumin also reduces inflammation and oxidative
damage, two factors that are also common contributors to heart
disease. One study on 121 people—all of whom were undergoing
coronary artery bypass surgery—found that the group taking 4
grams of curcumin for a few days before and after the surgery
were much less likely to experience a heart attack. Other
studies have revealed that the anti-inflammatory action of
turmeric helps prevent artery disease. Valdez points out that
recent studies suggest curcumin can protect the heart from
ischemia—an inadequate blood supply to an organ or part of the
body, particularly the muscles within the heart. A study
published in the journal Drugs in R & D found that a
standardized preparation of curcuminoids from Turmeric compared
favorably to the drug atorvastatin (trade name Lipitor) on
endothelial dysfunction, the underlying pathology of the blood
vessels that drives atherosclerosis, in association with
reductions in inflammation and oxidative stress in type 2
diabetic patients. Curcumin capsules were found to enhance the
functioning of endothelium lining in the heart’s blood vessels.
Any abnormality in the endothelial functioning can cause blood
pressure or cause blood clotting. This dysregulation then leads
to heart disease. Recent research literature on Curcumin
supplement intake suggest its treatment potential on par with
the drug Atorvastatin or regular moderate exercise. Moreover,
the already proven benefits of Curcumin/Turmeric in terms of
their anti-inflammation and anti-oxidation properties has a
benign influence on the heart as well. Even coronary artery
bypass surgery patients were found to have a significant
decreased risk of suffering a relapse heart attack upon starting
a Curcumin capsule regimen. curcumin administration (4 g/day
beginning from 3 days before the surgery and continued up to 5
days after surgery) significantly attenuated myocardial
infarction associated with coronary artery bypass grafting via
antioxidant and anti-inflammatory effects (Wongcharoen et al.
2012). In animal study, curcumin sup- plementation (10, 20 or 30
mg/kg) significantly reduced oxidative stress, apoptosis and
infract size via stimulating janus kinase 2/signal transducer
and activator 3 of transcription (JAK2/STAT3) signaling pathway
thus protects myocardium in ischemia reperfusion rats (Liu et
al. 2017). In another study, curcumin administration (150 mg/kg)
downregulated the NF-jB expression, upregulated PPAR-cand Bcl-2
expression, thereby attenuated apoptosis and inflammation in
rats with myocardial infarction injury (Lv et al. 2016).
Curcumin is reported to protect hypoxia-induced cardiomyocytes
apoptosis via downregulation of specific protein 1 (SP1) and
upregulation of miR-7a/b expression in mice (Geng et al. 2016).
It is known to reduce fibrosis by activating cardiac
NAD-dependent deacetylase sirtuin (SIRT)-1 expression during
myocardial infarction in mice (Xiao et al. 2016). Curcumin
treatment inhibited the activity of MMPs, reduced MDA level,
restored extracellular matrix degradation and decreased
deposition of collagen in ischemic/ reperfused myocardium of
rats. In addition, curcumin supplementation downregulated
phospho-Smad2/3 and TGFb1 expression while upregulated mothers
against decapentaplegic homolog 7 expression in the infarcted
myocardium, which might prove to be effective for the management
of heart attack (Wang et al. 2012). In in vitro study, curcumin
attenuated apoptosis and induce autophagy by upregulating Bcl-2
and downregulating the expression levels of beclin-1, Bax, SIRT1
and Bcl2/adenovirus E1B 19 kDa protein-interacting protein 3
(BNIP3) in hypoxia reoxygenation-induced H9c2 myocytes (Huang et
al. 2015b). These findings revealed that curcumin reverses
myocardial infarction and heart attack via its antioxidant,
anti-inflammatory and anti-apoptotic properties.
|
| How may
Curcumin work against kidney disease? |
| Kidney disease is a
condition in which the kidneys lose the ability to balance
fluids and eliminate waste. In animal model, curcumin treatment
significantly reduced plasma MPO activity, thiobarbituric acid
reactive substances (TBARS) level, superoxide anion generation
while increased GSH levels in rat ischemia reperfusion model of
acute kidney injury. In addition, curcumin reduced plasma
potassium level, plasma uric acid level, microproteinuria and
blood urea nitrogen along with induced NMDA receptor antagonism
during acute kidney injury resulting in nephroprotective effect
(Kaur et al. 2016). Curcumin administration (200 mg/ kg, p.o.)
significantly reduced the level of MPO, IL-1b, IL-6, IL-10,
TNF-a, MDA and caspase-3 resulting in protective effect against
cisplatin induced renal dysfunction in male Wistar albino rats
(Topcu-Tarladacalisir, Sapmaz-Metin, and Karaca 2016). Curcumin
administration downregulated the expression of NAD(P)H oxidase
subunits (p22phox, p47phox and p67phox), cytochrome P450 2E1
(CYP2E1) and nitrotyrosine renal protein. In addition, curcumin
decreased inflammatory cytokine like IFNc, IL-1band TNF- a.
Besides, the expression of glucose regulated protein 78, MAPKs,
p-ERK1/2, p-JNK and C/EBP homologous protein (CHOP) were
downregulated. In the same study, curcumin administration
reduced apoptosis signaling proteins (cleaved caspase-12 and
cleaved caspase-3) in low-dose streptozotocin with high-fat diet
induced nonalcoholic steatohepatitis kidney disease in mice
(Afrin et al. 2017). Curcumin ameliorated kidney function via
reducing plasma adiponectin, plasma sclerostin, plasma cystatin
C while increasing renal CAT, SOD, Nrf2, GSH in adenine induced
chronic kidney Figure 4. Modulation of growth factor pathways
and intracellular signaling components by curcumin in its
anticancer effects. Curcumin treatment blocked the effect of
Shh-Gli1, Wnt/b-catenin, ATKs and AR pathways as well as its
downstream signaling components which lead to reduce cancer
incidence, cancer progression, treatment resistance and disease
relapse (Ali et al. 2018). Moreover, curcumin administration
reduced renal mesangial matrix expansion, reduced renal
hypertrophy, downregulated fibronectin and collagen IV
expressions, decreased the levels of NLRP3 protein, cleaved
caspase-1 and IL-1bin the renal cortices of db/db mice (Lu et
al. 2017). Curcumin treatment reduced fibrosis of kidney by
decreasing the methylation of CpG in the klotho promoter,
resulting in induction of klotho expression and inhibition of
TGF-bsignaling in cyclosporine A induced mouse model of kidney
disease (Hu et al. 2016). In earlier study, curcumin
administration reduced superoxide production,
nicotinamide-adenine dinucleotide phosphate oxidase 4 level,
carbonylation of protein, nitrotyrosine -protein level,
autophagy and mitochondrial fission while increased GSH/ GSSG
ratio which leads to reversal of nephrotoxicity induced by
maleate treatment in rats (Molina-Jij on et al. 2016).
Experimental data have conclusively proved that, curcumin
treatment reduces fibronectin and collagen IV expressions,
suppresses TGF-bsignaling and exhibits antioxidant,
anti-inflammatory and anti-apoptotic potential thereby
ameliorating kidney functions. |
| How may
Curcumin work against skin disorders such as Psoriasis and
Dermatitis? |
| Curcumin has
anti-inflammatory, anti-oxidative and immunomodulatory effects,
and can inhibit T cell activation, proliferation and production
of pro-inflammatory factors by acting on MAPKs, AP-1, NF-κB
pathways. In peripheral blood mononuclear cells of
psoriasis stimulated in vitro, curcumin can effectively inhibit
T cell proliferation, proinflammatory cytokines and
multifunction, and inhibit T cell production of IFN-γ, IL-17,
Granulocyte-macrophage colony stimulating factor (GM-CSF) and
IL-22. Curcumin down-regulation pro-inflammatory cytokines then
inhibits the proliferation of imiquimod-induced differentiated
HaCaT cells. Vascular endothelial growth factor transgenic mice
can be used as a model to study psoriasis. Because in the
transgenic rat model of keratin (K) 14-VEGF, the inflammatory
skin condition has psoriasis-like cellular and molecular
characteristics, including characteristic vascular changes and
epidermal changes. Cytokine levels of TNF-α, IFN-γ, IL-2, IL-12,
IL-22 and IL-23 were reduced to normal level after curcumin
treatment. This may be due to the curcumin inhibits currents of
Kv1.3 channel and thus inhibits proliferation of T cells, or
curcumin influence MAPKs, AP-1 and NF-κB signaling pathways in
the psoriasis mice. Furthermore, research shows that curcumin is
capable of relieving TPA-induced inflammation by directly
down-regulating IFN-γ production. In an imiquimod-induced
psoriasis model, curcumin could alleviate inflammation
symptoms; lower TNF-α, IL-17A, IL-17F, IL-22, and IL-1β mRNA
levels; and lower CC Chemokine receptor 6(CCR6) protein
expression. Curcumin has a variety of mechanisms for
psoriasis, curcumin can keep dendritic cells in immaturity, to
accelerate the anti-inflammatory macrophage phenotype
polarization, inhibiting proinflammatory factor and T cell,
restrain the vascular endothelial growth factor, effect on
psoriasis susceptibility genes, and so on, has great potential.
Curcumin is derived from natural plant ingredients, which has
good safety and can be used for a long time without causing
serious toxic and side effects. Considerable evidence, both in animals
and humans, indicates that curcumin may be effective against
psoriasis. One study investigated whether the anti-psoriatic
activity of curcumin in patients is due to suppression of PhK
activity. The authors of this study concluded that drug-induced
suppression of PhK activity is associated with resolution of
psoriatic activity and that the anti-psoriatic activity of
curcumin may be achieved through modulation of PhK activity was also found to reduce wound-healing time,
increase collagen growth and increase blood flow to the skin.
Curcumin effectively
reduced the level of phosphorylase kinase in the skin of
psoriatic subjects. In addition, the effectiveness of curcumin
to reduce phosphorylase kinase level was more pronounced than
calcipotriol (Thangapazham, Sharma, and Maheshwari 2007).
Clinically, oral curcumin administration (20 mg, p.o., b.i.d.)
reduced the level of serum IL-22 and alleviated psoriasis
vulgaris (Antiga et al. 2015). In animal study, curcumin
administration (40 mg/kg, for 20 day) exhibited significant
reduction in ear thickness, ear weight, ear redness and lymph
node weight in the keratin 14-VEGF transgenic mouse model of
psoriasis. Furthermore, curcumin treatment downregulated the
serum levels of IL-2, IL-12, IL-22, IL-23, IFN-cand TNF-ain
psoriatic mice. Curcumin administration inhibited Kv1.3 channel
and suppressed the cytokines expression and T cells
proliferation resulting in reduction of psoriasis phenotype
(Kang et al. 2016). Curcumin treatment decreased incrassation
and skin inflammation in mouse ear induced by imiquimod.
Curcumin application promoted epidermal TCR cd-cell
proliferation and downregulated C-C chemokine receptor type 6
expression in the ear skin of imiquimod-induced psoriasis (Sun,
Zhao, and Hu 2013). Curcumin reduces psoriasis-associated
inflammation as well as hyper- proliferation of keratinocyte
that suggest its role in development of antipsoriatic drug
(Aggarwal, Surh, and Shishodia 2007).
This study on curcumin and skin found it is highly beneficial
for scleroderma, psoriasis and skin cancer. Dermatitis,
also called as eczema, is a group of disease that describes the
inflammation of skin. The polyphenol curcumin has been
traditionally used by Asian countries to manage dermatitis
(Gupta, Kismali, and Aggarwal 2013a). In a randomized,
double-blind, placebo-controlled study, curcumin administration
(6g/day, p.o., t.i.d, during radiotherapy) was reported to
reduce the dermatitis severity in breast cancer patients (Ryan
et al. 2013). In animal model, curcumin treatment reduces the
inflammation of mouse epidermis by reducing the activity of
epidermal COX and lipoxygenase (LOX). The biological effect of
curcumin to reduce dermatitis is mainly due to inhibition of COX
and LOX activities. A phase II, open-label, Simon’s two-stage
clinical trial sought to determine the safety and efficacy of
oral curcumin in patients with moderate to severe psoriasis.
Twelve patients with chronic plaque psoriasis were enrolled in
the study and were given 4.5-g curcumin capsules every day for
12 weeks, followed by a 4-week observation period. Curcumin was
well-tolerated, and all participants completed the study.
Patients who responded to the treatment showed 83% to 88%
improvement at 12 weeks of treatment. |
| How may
Curcumin work against Endocrine disorders such as Osteoporosis,
Hypothyroidism, and Hyperthyroidism? |
| Curcumin administration
ameliorated microarchitecture of tibia bone through
down-regulation of MMP-9 expression, inhibition of
osteoprotegerin (OPG)/RANK ligand/RANK signaling and the
activation of microRNA-365 in dexamethasone treated mice (Li et
al. 2015a). It has been indicated that MiR-365 act as an
upstream regulator of MMP-9 during osteoporosis. Mechanistically
curcumin treatment ameliorated bone deteriorations through the
activation of miR-365 via suppressing MMP-9 (Li et al. 2015a).
One study revealed that, curcumin administration increased the
ratio of osteoprotegerin to receptor activator for NF-kB ligand,
ameliorated the proliferation of osteoblasts and activated the
Wnt signaling thereby alleviated osteoporotic symptoms induced
by glucocorticoid in rats (Chen et al. 2016). Curcumin treatment
(100 mg/kg for 2 month) increased bone mineral density,
downregulated the ratio of Bax/Bcl-2, downregulated cleaved
poly-ADP-ribose polymerase (PARP) and cleaved caspase-3,
upregulated p-ERK1/2 expression as well as reduced femoral
osteoblast apoptosis in glucocorticoid-induced osteoporosis rat
model (Chen et al. 2016). Recently, report suggests that
curcumin reversed hind-limb suspension-induced bone loss in rats
via upregulation of vitamin D receptor expression and
attenuation of oxidative stress (Xin et al. 2015). In in vitro
studies, curcumin treatment ameliorates the viability of
Saos-2 cells, reduces apoptosis, improves the mitochondrial
membrane functions and its potential, upregulates GSK3b and
protein kinase B (Akt) phosphorylation. These evidences of
curcumin administration supporting its potential for management
of osteoporosis (Dai et al. 2017). Curcumin reduce the risk of
osteoporosis via several mechanisms including reduction of
apoptosis, amelioration of mitochondrial membrane function, PKB
phosphorylation, microRNA-365 activation and osteoblasts
proliferation. upregulated expression of hepatic glutathione
reductase, GPx-1 and CAT were mitigated by concomitant
administration of curcumin and vitamin E in 6-propyl-thio-uracil
induced hypothyroid rats. In addition, curcumin and vitamin E
supplementation reduced the enhanced activity of MnSOD-2, GPx-1
and suppressed activity of glutathione reductase in
mitochondrial fraction. It was concluded that curcumin and
vitamin E supplementation modulate hepatic antioxidant gene
expression during hypothyroidism (Subudhi and Chainy 2012).
Curcumin administration significantly reduced the level of LPO
in cerebellum and cerebral cortex of
6-propyl-2-thiouracil-induced hypothyroidism in rats. In
addition, curcumin reversed the decreased level of translated
products SOD1 and SOD2 in rats with hypothyroidism (Jena et al.
2012). Interestingly, an earlier study suggested that, vitamin E
and curcumin administration restore the activity of serum
transaminase, altered rectal temperature and hepatic
histoarchitecture in rats with hypothyroidism induced by
6-n-propyl-2-thiouracil (Subudhi et al. 2009). Hyperthyroidism
In animal study, curcumin administration reduced lipid
peroxidation in the cerebral cortex of l-thyroxine induced
hyperthyroid rats. Interestingly, curcumin reduced the activity
of SOD, SOD1 and SOD2 in cerebral cortex, while enhanced the SOD
and SOD1 activity in the cerebellum of hyperthyroid rat (Jena,
Dandapat, and Chainy 2013). In another study, curcumin and
vitamin E administration reversed the reduced levels of hepatic
SOD and CAT. Besides, curcumin administration upregulated the
expression of glutathione peroxidase-1 and glutathione reductase
in rat liver. In the same study, co-treatment of curcumin along
with vitamin E alleviated oxidative stress and liver damage in
l-thyroxine induced hyperthyroid rats (Subudhi and Chainy 2010).
Further, l-thyroxine induced hyperthyroidism and its associated
increase in activity of ALT and AST in rat serum were reduced by
curcumin and vitamin E treatment resulting in hepatoprotection
(Subudhi et al. 2008). These finding suggest that, curcumin
administration exerts neuromodulatory and hepatoprotective
activity during hyperthyroidism mainly due to its antioxidant
effect. |
| How may
Curcumin work against Respiratory disorders such as asthma,
pulmonary disease, Pneumonia, and allergies? |
| Curcumin inhibited the degranulation
and release of histamine from rat peritoneal mast cells caused
by compound 48/80. In an animal model, curcumin dramatically
reduced the mast cell-mediated passive cutaneous anaphylactoid
reaction. Curcumin enhanced intracellular cAMP levels and
inhibited both nonspecific and selective mast cell-mediated
allergy reactions (Choi et al., 2010). Curcumin significantly
reduced IgE/Ag-induced PSA (passive systemic anaphylaxis), as
measured by serum-dependent leukotriene C4, dependent
prostaglandin D2, and histamine levels, indicating that it might
be useful to produce drugs for allergic inflammatory illnesses
(Li et al., 2014). Curcumin can suppress expression of CD80,
CD86, and class II antigens by dendritic cells and blocks the
release of inflammatory cytokines like IL1β, IL-6, and TNF-α
from LPS-stimulated dendritic cells. Chronic obstructive
pulmonary disease (COPD) is a progressive airflow limitation
disease associated with persistent inflammation of respiratory
system, especially in the airways and lungs. COPD is caused by
long-term exposure with noxious particles, gases, such as air
pollutants or smoke. The preclinical studies in animal model,
there are anti-inflammatory effects of curcumin can reduce and
alleviate respiratory inflammation and oxidative stress caused
by exposure to soot or other air pollutants. Curcumin also
reduces allergic asthma by inhibiting the PPARγ/NF-κB signaling
pathway in respiratory mucosa to prevent COPD. When lower
airways and lung parenchyma with severe acute lung injury or
inflammation by drugs or infection may cause plasma
extravasation, which leads to lung infiltration filled with
interstitial fluid and leukocytes. During the recovery from
severe lung infiltration, some patients will develop fibrous
tissue hyperplasia and pulmonary fibrosis, resulting in a
significant reduction in lung gas exchange area and efficiency.
For example, clinical application of chemotherapeutic drugs or
radiotherapy can cause acute lung injury and lead to pulmonary
fibrosis and curcumin treatment can attenuate the severity of
pulmonary fibrosis. There are several inflammatory cytokines
related with pulmonary fibrosis, include TNF-α, TNF-β1, IL-6,
and IL-4, ROS, MMPs, and TGF-β, and treated with curcumin can
inhibit those cytokine expressions. Today's most famous
coronavirus, patients with SARS virus and COVID-19 infection
will often have sequelae of severe pulmonary fibrosis after
healing from viral infection, and curcumin also can act on the
spike protein to interrupt the covid-19 infection. Many clinical
studies on pulmonary fibrosis have pointed out that curcumin or
turmeric can significantly reduce the expression of
inflammation-related factors in cells and tissues, effectively
moderates excessive inflammation in the acute phase of lung
injury or infection, and prevents pulmonary fibrosis in the
later stage. Asthma is a chronic lung
disease involving the inflamed, swell and narrowed airways that
produce extra mucus, which causes breathing difficulties.
Clinically, curcumin administration (500 mg/day for 30 days)
ameliorated the mean forced expiratory volume one second values
resulting in alleviation of airway obstruction alongside
improved haematological parameters in asthmatics (Kunnumakkara
et al. 2017). In animal study, intranasal curcumin
administration attenuated the pulmonary fibrosis and
inflammation of airway by downregulation of MMP-9, eotaxin,
TIMP-1 and a-smooth muscle actin expressions in the lung tissue
of ovalbumin-induced chronically asthmatic mice (Chauhan,
Dash, and
Singh 2017). In another study, curcumin administration reduced
inflammatory markers like IL-4 and INF-c levels in lung tissue
alongside reduced asthma symptoms by activation of Wnt/b-catenin
signaling pathway in ovalbumin challenged mice (Yang et al.
2017c). Further, curcumin administration suppressed the
activation of JNK54/ 56, ERK 42/44 and p38 MAPK resulting in
inhibition of COX-2 expression and prostaglandin (PG) D2
release, which is known to reduce airway obstruction,
inflammation and asthma progression in ovalbumin challenged
mouse model of asthma (Chauhan et al. 2016). Evidence suggested
that lipopolysaccharide exposure causes increase in level of
IgE, IL-4, IL-5, histamine and MPO resulting in exacerbation of
airway inflammation in rats and these effects were efficiently
reversed by curcumin administration (Kumari, Dash, and Singh
2015). Curcumin treatment is reported to attenuate the
production of IgE, accumulation of inflammatory cells and
hyperplasia of goblet cell alongside ameliorated the secretion
of mucus and hyper-responsiveness of airway in asthmatic mice. In
addition, curcumin administration increased the activity of HO-1
and Nrf-2 while reduced p-IjB and NF-jB levels in the lung
tissue of ovalbumin challenged female specific pathogen-free
BALB/c mice (Liu et al. 2015). Their mechanism of action is
associated essentially due to its anti-oxidative and
anti-inflammatory activities in asthma. At molecular and
cellular levels, curcumin treatment reduces asthma symptoms
mainly due to inhibition of histamine release, attenuation of
IgE, inhibition of COX-2 enzyme and suppression of JNK54/56, ERK
42/44 and p38 MAPK activation (Chauhan et al. 2016;He et al.
2015c). Chronic obstructive pulmonary disease (COPD) is a
progressive inflammatory lung disease that causes obstruction in
airflow and difficulty in breathing. In animal study, curcumin
administration is known to ameliorate right ventricular
hypertrophy index and right ventricular systolic pressure via
activation of suppressor of cytokine signaling (SOCS) 3/
JAK2/STAT signal transduction in lung tissue of rat with chronic
obstructive pulmonary disease (Lin, Chen, and Liu 2016).
Curcumin treatment downregulated macrophage inflammatory protein
(MIP)-2a, IL-8 and MCP-1 expressions while upregulated histone
deacetylase 2 expression, ameliorated methylation of H3K9 and
reduced H3/H4 acetylation in type II alveolar epithelial cells
during cigarette smoke exposure induced chronic obstructive
pulmonary disease in rats (Gan et al. 2016). Further, it was
reported that, curcumin administration reduce TNF-a, IL-6, IL-8
level, macrophages count, neutrophil numbers and total cell
numbers alongside reversed ultrastructural damage and emphysema in bronchoalveolar lavage fluid of cigarette smoke exposure
combined intratracheally administered lipopolysaccharide induced
chronic obstructive pulmonary disease in rats. Additionally,
curcumin downregulated alveolar epithelia p66Shc and p-p66Shc
expression, which is associated with protection of alveolar
epithelial injury (Zhang et al. 2016c). We conclude that
curcumin suppresses the progression of chronic obstructive
pulmonary disease by inhibiting the inflammation of airways.
These findings suggest that curcumin could be used to protect
chronic obstructive pulmonary disease in human and animals.
Pneumonia is an inflammatory condition caused by
bacteria, viruses or fungi in one or both lungs. In animal
model, curcumin treatment reduced pneumonia in female C57BL/6J
mice caused by Staphylococcus aureus via inhibiting the
pore-forming activity of a-hemolysin, an extracellular protein
secreted by bacteria that is known to induce the lung infection
(Wang et al. 2016a). Further, curcumin significantly reduced S.
aureus-mediated lung edema, barrier disruption, vascular leakage
and pneumonia. In addition, curcumin administration
significantly reduced neutrophils infiltration and attenuates
plasminogen activator inhibitor-1 activation, resulting in
reduction of chemokines and cytokines in staphylococcus
aureus-infected mouse model of pneumonia (Xu et al. 2015). Thus,
continued studies of the potent anti-inflammatory,
anti-microbial, anti-oxidant agent, curcumin, will likely use to
reverse or slow the progression of pneumonia, ultimately,
leading to novel treatments for pulmonary dysfunction in
critically ill patients (Avasarala et al. 2013). Allergies, also
known as allergic diseases, are a number of conditions in which
the immune system reacts abnormally to a foreign substance. In a
randomized, double-blind study, chronic curcumin administration
(500 mg/day, p.o., for consecutive 2 months) significantly
alleviated rhinorrhoea, sneezing and nasal congestion in
patients by reducing nasal airflow resistance. In addition,
curcumin administration suppressed TNF-a, IL-4 and IL-8, while
increased the production of soluble intercellular adhesion
molecule and IL-10 (Wu and Xiao 2016). Curcumin administration
(2.5 or 5 mg/kg, for four days) suppressed the level of IgE in
the serum of asthmatic mice. Further, it reduced the level of
secretory phospholipase A2, COX-2, nitric oxide, IL-4 and IL-6
in bronchoalveolar lavage fluid. In addition, curcumin
administration downregulated the expression of p38, COX-2, p-ERK
and p-JNK in the lungs tissue of ovalbumin challenged mice
(Chauhan et al. 2016). Study revealed that curcumin treatment
significantly reduce histamine release and downregulate TNF-a,
IL-1b, IL-6, IL- 8, p-ERK, p-p38, p-JNK, p-IkBaand NF-kB p65
expressions in mast cells. Besides it decreased the levels of
IgE, histamine, TNF-a, Src kinases, Fyn, Lyn and Syk in the
serum of mice with allergic rhinitis induced by ovalbumin (Zhang
et al. 2015b). Curcumin supplementation significantly attenuated
lipopolysaccharide induced allergic asthma by reducing airway
inflammation and decreasing IgE level, histamine release and
oxidative stress in mice (Kumari, Dash, and Singh 2015).
Further, curcumin administration inhibited intestinal
mastocytosis, expression of Th2 cytokines, intestinal
anaphylaxis and activation of NF-jB in ovalbumin. These findings
suggest that, the anti-allergic mechanism of curcumin is
essentially due to its anti-inflammatory and anti-oxidative
activities. At cellular and molecular levels, curcumin treatment
reduces allergic symptoms mainly due to attenuation of IgE,
inhibition of histamine release, inhibition of COX-2 enzyme,
stimulation of Nrf- 2/ HO-1 pathway etc. (Chong et al. 2014;
Kurup and Barrios 2008; Lee et al. 2008). |
| What are the scientific properties of Curcumin? |
|
Chemical Name |
Diferuloylmethane |
|
Definition |
A β-diketone that is methane in which two of the hydrogens are substituted by feruloyl groups |
|
Systemic Name |
(1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) |
|
Empirical Formula |
C21H20O6 |
|
Linear Formula |
[HOC6H3(OCH3)CH=CHCO]2CH2 |
|
Molecular
Weight |
368.38 g/mol |
|
Appearance |
Bright yellow-orange powder |
|
Melting Point |
183 °C, 361 °F |
|
PubChem |
969516 |
| Biofunction |
Enzyme cofactor |
|
Chemical Taxonomy |
Organic Chemicals / Hydrocarbons / Aromatic
Compounds / Phenols / Catechols / Curcuminoids |
|
Chemical Structure |
 |
| Taxonomic hierarchy |
Kingdom
Subkingdom
Superdivision
Division
Class
Subclass
Order
Family
Genus
Species |
Plantae
(Plants)
Tracheobionta (Vascular plants)
Spermatophyta (Seed plants)
Magnoliophyta (Flowering plants)
Liliopsida (Monocotyledons)
Zingiberidae
Zingiberales
Zingiberaceae (Ginger family)
Curcuma (Curcuma)
Curcuma longa (Turmeric) |
|
|
Why may curcumin extracted
from Turmeric root provide health benefits?
|
|
Natural plant products
have been used throughout human history for various purposes.
Having co-evolved with animal life, many of the plants from
which these natural products are derived are billions of years
old. Natural polyphenol compounds derived from these plants, such
as curcumin, have many favorable biological properties. Plants manufacture chemicals that repel
predators, herbivores, parasites and diseases, and these chemicals
are produced as secondary metabolites as a
natural defense mechanism. In particular, curcumin, a
polyphenolic compound, is synthesized in the rhizome root of
Curcuma longa, or turmeric. This process involves several
enzymatic steps within the plant. It starts with phenylalanine
ammonia-lyase converting phenylalanine into cinnamic acid, a
critical step in the biosynthesis of phenylpropanoids, of which
curcumin is a part. Cinnamic acid then undergoes a series of
enzymatic reactions in which p-coumaric acid acts as a key
intermediate compound, forming curcumin precursors known as
curcuminoids. These precursors, including curcumin,
demethoxycurcumin, and bis-demethoxycurcumin, are further
modified through a series of enzymatic reactions. These
reactions lead to the formation of molecules commonly known as
curcumin, the main active compound responsible for turmeric’s
therapeutic activity and characteristic yellow-orange color.
This biosynthesis and accumulation process occurs in the
rhizomes of the turmeric plant, where it serves various
functions, including protection against oxidative stress and
defense against pathogens. Many of
these natural chemicals have pharmacological or biological
activity, and these medicinal plants have played a pivotal role
in the health care of many cultures, both ancient and modern.
Medicinal plants and their bioactive ingredients, in particular
polyphenols, have been implicated in various biological
activities, including, but not limited to, immunomodulation,
anti-inflammatory, cardiovascular protection, antioxidant, and
anticancer potential. Like most of these pharmacologically-active metabolites,
curcumin is involved in self-defense. Over time, plants with higher levels of
organic compounds that deter attackers become more successful, because of their
advanced protection. In nature's never-ending interaction between predator and prey,
insects evolve the ability to digest plant toxins, while plants evolve stronger chemicals to deter their enemies. Monitoring
this evolution between plants and insects represents an important field of
ecological research. Scientists have discovered that many phytochemicals manufactured
in plants and roots not only prevent insect attack or fight plant infections, but also provide
human health benefits.
Many cultures create their own botanical pharmacies as
the lore of medicinal plants and remedies is handed down through generations of
healers. Medicinal plants have been used as a traditional treatment agent for
numerous human diseases since ages in many parts of the world.
In rural areas of the developing countries, they continue to be
used as the primary source of medicine. With the advent
of sophisticated laboratory testing, biologists are finding that the many
indigenous plants and roots from around the world provide medicinal
value, and their metabolites are candidates for research. Pharmaceuticals, nutraceuticals, phytotherapeutics and herbal medicines are found thanks to
ethnobotany and developed by researching and analyzing compounds
derived from plants. Turmeric derived from the
Curcuma longa plant
contains the polyphenols curcumin, demethoxycurcumin, and
bisdemethoxycurcumin, and it has caught the attention of
researchers due to its extensive use as a culinary ingredient
(the bright yellow color of curry is attributed to turmeric) in
most Asian countries and the many reports of its antioxidant,
antimicrobial, and anti-inflammatory properties. Curcumin
(diferulolylmethane) is extensively utilized in a variety of
settings including cosmetic and herbal supplementation, and its
medicinal properties have been investigated for more than 50
years. Curcumin’s structure is similar to other natural
polyphenolics (chemicals containing multiple "phenol" groups)
produced by plants in response to infectious attack. These
natural polyphenols often have potent antioxidant and
anti-inflammatory properties as well as immune support health
benefits. Every medicinal molecule in all of
botany is made out of carbon dioxide. CO2 is the key source of carbon used by
plants to synthesize everything from curcumin to resveratrol. Every healing
nutrient in basil, oregano, cinnamon, turmeric, garlic and pomegranate fruit is
made out of carbon dioxide. Curcumin, a powerful anti-cancer nutrient found in
turmeric, is made from carbon dioxide, with 21 carbon molecules manufactured
from atmospheric carbon dioxide. Curcumin has been
studied for antioxidant, anti-inflammatory,
antiviral, antibacterial, antifungal, and anticancer activities, mediated
through the regulation of various transcription factors, growth factors,
inflammatory cytokines, protein kinases, and other enzymes. Curcumin exhibits
activities similar to recently discovered TNF blockers (humira, remicade and
enbrel), vascular endothelial cell growth factor blocker (avastin), human
epidermal growth factor receptor blockers (erbitux, erlotinib, and geftinib),
and HER2 blocker (herceptin).
With
antioxidant, anti-inflammatory and antitumor properties,
curcumin has received much attention in several
neurodegenerative diseases, such as Alzheimer’s (AD), Huntington
(HD) and Parkinson’s diseases (PD). Extensive research over
several decades has sought to identify the mechanisms of
molecular action of curcumin. It regulates inflammatory
cytokines, growth factors, growth factor receptors, enzymes,
adhesion molecules, proteins related to apoptosis and cell-cycle
proteins, such as cyclin, and modulates its molecular targets by
altering their gene expression, signaling pathways or through
direct interaction.
Considering the recent scientific bandwagon that
multi-targeted therapy is better than mono-targeted therapy for most diseases,
curcumin is a phytonutrient that can be considered an ideal "Spice for Life". More than 5000
papers published within the past two decades have revealed that curcumin has
extraordinary potential in promoting health through modulation of numerous molecular
targets. The importance of curcumin can be estimated by the fact that
thirty-seven cases of clinical trials of curcumin were
completed by December 2017 and FDA (Food and Drug
administration) clinical phase 4 trials have been completed. |
| Why hasn't
the pharmaceutical industry patented Curcumin? |
|
Pharmaceutical
corporations tried registering patents for curcumin and
turmeric because of the much heralded scientific
evidence and the long history of its healing properties.
However, that same
evidence and history of
curcumin being used medicinally for centuries was the reason the United
States Patent and Trademark Office rejected and revoked the rights
for turmeric patent 5401504
on the grounds that the claims were
not new: "USPTO
unequivocally rejected all six claims made on August 13, 2001 while ruling that Turmeric's
medicinal properties were not patentable." University of Texas MD
Anderson Cancer Center states
"in
the case of curcumin, a natural compound, no company can reap the benefits if
turmeric shows itself to be an effective anti-cancer drug."
And because curcumin is not economically interesting,
"it is almost impossible to get financial support to conduct a
clinical trial with a substance that cannot be patented. The
greatest challenge will be to find sponsors for clinical
research on curcumin, as this promising plant-derived compound
cannot be exploited economically." |
|
What is the history of Curcumin and Turmeric?
|
Turmeric | Turmeric boasts a rich history of
use as an herbal medicine, spice, and coloring agent. Records
dating back to 600 BC in an Assyrian herbal book, references by
the renowned Greek physician Dioscorides, and mentions in
Islamic traditional medicine contribute to its historical
significance. Turmeric is integral to Chinese traditional
medicine, Ayurveda, and various folk medicines worldwide.
According to Sanskrit medical treatises and Ayurvedic and Unani
systems, turmeric has a long history of medicinal use dating
back to the Vedic period (ca. 1500–ca. 500 BC). It is featured
in the Suśrutasaṃhitā, the foundational text of traditional
Indian medicine, as an ingredient to alleviate the effects of
poisoned food (Prasad, 2011). Curcuma Longa originated
in India and has a history of 6000 years. The recorded use of
turmeric dates back nearly 4000 years to the Vedic culture in
India, where it was used as a culinary spice and had some
religious significance. The use of turmeric is most salient in
the various island cultures of the Pacific, where it spread with
the Austronesian expansion there starting around 3000 BC,
reaching as far as Polynesia and Fiji (McClatchey, 1993; Prance
& Nesbitt, 2005; Sopher, 1964). Susruta’s Ayurvedic Compendium,
dating back to 250 BC, recommends an ointment containing
turmeric to relieve the effects of poisoned food. Researchers in
India recently identified mineral remnants of turmeric on the
cooking pots of ancient Indus River remains, one of the first
urban civilizations. These ancient civilizations have vast trial
and error experience with many different herbal remedies and
food preparations and they selected curcumin for medicinal use
based on efficacy. The name turmeric
derives from the Latin word terra merita (meritorious
earth), referring to the color of ground turmeric which
resembles a mineral pigment. The Arabic word for turmeric is
kurkum, which originally meant ‘saffron.’ The word kurkum
in Hebrew is karkom as it is written in the Bible. Medieval Arabic
literature called turmeric zaʿfarān hindī
which means "Indian saffron". According
to Nair (2019, p. 2), its maritime dispersion from India
intensified in the Middle Ages, reaching the coast of China in
the seventh century AD, East Africa a century later, West Africa
by 1200, and Jamaica in the eighteenth century. In Chinese
medicinal literature, turmeric first appears in the Xinxiu
Bencao, and the Bencao Gangmu treats it as well (Feng
et al., 2011). From its initial diffusion up to Vasco da Gama’s
journey and landing in Kozhikode, it was Arab traders who were
instrumental in the westward spread of turmeric, similarly to
pepper and other spices of the time. In 1280, Marco Polo
described turmeric in the China leg of his travels; "There is
also a vegetable which has all the properties of the true
saffron, as well the smell as the colour, and yet it is not
reall saffron. It is held in great estimation, and being an
ingredient in all their dishes, it bears, on that account, a
high price." -
The Travels of Marco Polo, the Venetian
Curcumin | Curcumin
is a golden yellow compound found in turmeric root and was first isolated in 1815 by two German
scientists, Vogel and Pelletier. They reported on the “yellow
coloring-material” from the rhizomes of the East Indian plant
Curcuma longa and named it
curcumin. In 1842,
they identified curcumin as the most abundant molecule and the
primary curcuminoid in turmeric.shed a new analysis of a purer
curcumin (melt ing-point 120?) ; but It was not till 1870 that
curcumin was obtained essentially pure by Ivanow-Gajewsky;
On
Certain Substances Obtained from Turmeric. I. Curcumin | 1881.
In 1910 the chemical structure of curcumin
was established as a diferuloylmethane, specifically
1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione, by
Miłobȩdzka J., Kostanecki S., Lampe V.
An early mention of curcumin in
modern medical literature was its first clinical trial,
appearing in the Lancet in 1937, one of the most prestigious
medical journals:
Turmeric (Curcumin) In Biliary Diseases. The article, describing curcumin applications
to humans, was written by Albert Oppenheimer—then an assistant
professor at the American University of Beirut, Lebanon, who
applied curcumin orally for the treatment
of 67 patients suffering from various forms of subacute,
recurrent, or chronic cholecystitis. The positive therapeutic
response recorded then, was the basis for future interest in
curcumin and its healing properties, especially its
anti-inflammatory properties, which were among the first
studied.
The first study on its biological activity as an
antibacterial agent was published in 1949 in Nature. Curcumin's
biological properties were described as responsible for most of
the therapeutic efficacy of turmeric in the 1970s when the first
research on curcumin’s health benefits was carried out. In 1985,
Kuttan et al first confirmed its anticancer effects;
Potential anticancer activity of turmeric (Curcuma longa) |
Cancer Letters. In these
and in later studies it was shown that curcumin has multiple
therapeutic potentialities (Di Mario et al., 2007; Adhvaryu et
al., 2008; Chandran and Goel, 2012; Yanpanitch et al., 2015;
Gera et al., 2017; Salehi et al., 2019a). Although
the current database indicates over 12,000 publications on
curcumin, until 1990 there were less than 100 papers published
on this nutraceutical.
In 2007,
Payton et al showed that curcumin's form exists in
solution as a keto−enol tautomer. Numerous
studies have indicated that curcumin is a highly potent
antimicrobial agent and has been shown to be active against
various chronic diseases including various types of cancers,
diabetes, obesity, cardiovascular, pulmonary, neurological and
autoimmune diseases. Furthermore, this compound has also been
shown to be synergistic with other nutraceuticals such as
resveratrol, piperine, catechins, quercetin and genistein.
Curcumin's
molecular identity is characterized by unique polyphenolic
elements, specifically two feruloyl groups linked via a
methylene chain. This molecular configuration plays a crucial
role in its biological and chemical properties. For generations,
curcumin has been a key component in the traditional medicinal
practices of various Asian cultures. Its use in Ayurvedic and
traditional Chinese medicine, along with other herbal systems,
has been extensive. Curcumin has been applied to treat a wide
range of ailments, from inflammation and pain to more specific
conditions such as jaundice, menstrual issues, bleeding, dental
pain, bruises, and cardiac pain. In the realm of
contemporary science, curcumin has sparked considerable interest
due to its potential health benefits. Studies have delved into
its effectiveness against chronic illnesses like cancer,
Alzheimer’s disease, heart diseases, and inflammatory conditions. This interest is fueled by its properties as an
antioxidant and an anti-inflammatory, and its possible role in
cancer prevention. Scientists are examining how curcumin
influences various cellular processes by interacting with
multiple signaling molecules, including growth factors,
cytokines, and the genes involved in cell life cycle and
division. The high degree of reverence
is established by
the fact that it is used in so many cultures: Arabic
Kurkum, Uqdah safra. Armenian
Toormerik, Turmerig. Assamese
Halodhi. Bengali
Halud. Bulgarian
Kurkuma. Burmese
Hsanwen, Sanwin, Sanae, Nanwin. Catalan
Cúrcuma. Chinese
yujin [yü-gold]Yu chin, Yu jin, Wohng geung, Geung wohng, Wat gam, Huang jiang,
薑黃
Jiang huang, Yu jin xiang gen. Croatian
Indijski šafran, Kurkuma.
Czech Kurkuma, Indický Šafrán,
Žlutý kořen, Žlutý zázvor.
Dhivehi Reen’dhoo. Danish
Gurkemeje. Dutch
Geelwortel, Kurkuma Tarmeriek, Koenjit, Koenir. English
Turmeric, Curcumin, Indian saffron. Estonian
Harilik kurkuma, Kurkum, Pikk kollajuur, Lŏhnav kollajuur,
Harilik kurkuma, Kurkum, Pikk kollajuur, Lŏhnav kollajuur. Farsi
Zardchubeh. Finnish
Kurkuma, Keltajuuri. French
Curcuma, Safran des Indes, Terre-mérite, Souchet des Indes. Galician
Cúrcuma. German
Curcuma, Sárga gyömbérgyökér.
Greek Kitrinoriza, Kourkoumi,
Kourkoumas Gujarati Halad, Haldar. Hebrew
Kurkum, Kurkume. Hindi
Haldi. Hungarian
Kurkuma, Sárga gyömbérgyökér.
Icelandic Túrmerik. Indonesian
Kunyit, Kunir, Daun kunyit.
Italian Curcuma. Japanese
Ukon, Tamerikku Kannada Arishina, Arisina. Khmer
Romiet, Lomiet, Lamiet. Korean
Kang-hwang, Keolkuma Kolkuma, Sim-hwang, Teomerik, Tomerik,
Tumerik, Ulgum, Ulgumun.
Laotian Khi min khun, Khmin
khÜn. Latvian
Kurkuma. Lithuanian
Ciberžole, Kurkuma, Dažine ciberžolé. Malay
Kunyit basah. Malayalam
Manjal Marathi Halad. Nepali
Haldi, Hardi, Besar. Norwegian
Gurkemeie Pahlavi Zard-choobag. Pashto
Zarchoba. Polish
Kurkuma, Ostryź długi, Szafran indyjski. Portuguese
Açafrão da Índia, Curcuma.
Punjabi Haldi. Romanian
Curcumǎ. Russian
Koren, kurkumy, Kurkuma.
Sanskrit haldi. Singhalese
Kaha. Slovak
Kurkuma. Slovenian
Kurkuma. Spanish
Cúrcuma, Azafrán arabe.
Swahili Manjano. Swedish
Gurkmeja. Tagalog
Dilaw. Tamil
Manjal. Telugu
Haridra, Pasupu. Thai
Kha min chan, Kha min; Wanchakmadluk. Tibetan
Gaser, Sga ser. Turkish
Hint safrani, Sari boya, Zerdeçal, Safran kökü, Zerdali,
Zerdeçöp, Zerdecube. Ukrainian
Kurkuma. Urdu
Haldi, Zard chub. Vietnamese
Bot nghe, Cu nghe, Nghe, Uat kim, Khuong hoang. |
|
What is Turmeric-Curcumin.com?
|
|
Turmeric-Curcumin.com
is our company and website, dedicated for over twenty four
years to researching, manufacturing and distributing the highest
quality curcumin 95% extract. Since our establishment in 2000,
we've been dedicated to offering the best customer service without the
typical marketing hype, allowing us to maintain the lowest
prices in the industry. Unlike merchants with numerous products and categories,
we've remained focused on
wholesaling and retailing the
single most beneficial compound in botanical
medicine; curcumin, standardized and
purified to 95% extract. This extraordinary herbal extract of
turmeric continues to
generate interest in universities and medical centers around the
world, with studies
and clinical trials exploring the many health benefits of the curcumin 95%
that we produce and supply. You will receive a
100% natural, additive-free product:
no
synthetics, starch, no sugars or sweeteners, no artificial
colors or flavors, no sodium, no soy, no yeast, no wheat, no
corn, no rice or other grains, no gluten, no dairy, no
preservatives, no gums, no dyes, and no GMO. For questions or comments, please
email service@turmeric-curcumin.com, call / text
206.339.7899
or mail
TURMERIC-CURCUMIN.COM
4031 Industrial Center Drive, North Las Vegas, NV 89031, USA. |
|
©
2000-2024 TURMERIC-CURCUMIN.COM
"The physician treats, but nature
heals" - Hippocrates,
400 BC |
| |